Introduction
Nanobiotechnology is one of the new technologies in the field of biological sciences. Nanoparticles (NPs) have molecular size of 1–100 nm. Several methods are used to synthesize NPs, such as physical, chemical, and biological methods. Physical methods include plasma arcing, vapor deposition, spray pyrolysis, plasma and ultrasonic irradiation, lithographic techniques, molecular beam epistaxis, and diffusion flame synthesis of NPs (Kong et al., 2001; Guan and Pedraza, 2008; Shoushtari, 2008; Ghaffarian et al., 2011; Krol et al., 2017). The chemical methods include precipitation, microemulsion, chemical reduction, sol-gel, and hydrothermal techniques (Brintha and Ajitha, 2015; Bekkari et al., 2017; Krol et al., 2017; Naveed et al., 2017). These techniques, however, require a high amount of energy and involve toxic and harmful chemicals, which may lead to health risks (Fakhari et al., 2019).Presently, biological methods for producing metal and metal oxide NPs are becoming increasingly popular as there is a huge inventory of resources that may be used to produce biological NPs, such as plants and plant products (Ahmad et al., 2010), algae (Azizi et al., 2014), fungi (Uzzaman et al., 2017), yeasts (Boroumand Moghaddam et al., 2017), bacteria (Kundu et al., 2014;Vahidi et al., 2019), and viruses (Nam et al., 2006). Various studies have suggested that plants are the best option to synthesize biological NPs (Wang et al., 2009). This might be because vegetal substrates are believed to be more cost-effective, easy to process, and less toxic than microorganisms. The use of plant-based substrates also does not involve exposure-related health risks or safety concerns related to hazardous microorganisms (Andeani et al., 2011; Ramesh et al., 2014). Among the metal oxide NPs, zinc oxide NPs have received great interest because of their vast application in various areas such as optical, magnetic, piezoelectric, and gas sensing (Gunalan et al., 2012; Fakhari et al., 2019). Previous studies have revealed that ZnONPs have strong pharmacological properties such as anticancer (Selvakumar et al., 2015; Anitha et al., 2018), antimicrobial (Soren et al., 2018; Safawo et al., 2018), antioxidant (Stan et al., 2016; Soren et al., 2018), wound healing, anti-inflammatory, and antidiabetic (El-Gharbawy et al., 2016; Agarwal et al., 2017) properties. Thus, researchers have been investigating low-cost, non-toxic and relatively simple methods to biologically synthesize ZnONPs (Das et al., 2017). Biological synthesis of ZnONPs using various plant extracts such as Aloe barbadensis miller leaf extract (Sangeetha et al., 2011), Cinnamonum camphora (Huang et al., 2007), Azadirachta indica neem, lemongrass (Dwivedi and Gopal, 2010), and tamarind (Ankamwar et al., 2005) has been reported. Antimicrobial activities of metal oxide powders (ZnO, MgO, and CaO) against Staphylococcus aureus and Escherichia coli have been quantitatively evaluated inculture media (Ankamwar et al., 2005). ZnONPs were shown to exhibit significant antimicrobial activity against both gram-positive and gramnegative bacteria (Raghupathi et al., 2011; Jiménez et al., 2015; Sharma et al., 2016; Jahanpanahiand and Ali Mohamadi, 2016;Vahidi et al., 2019).
Fumaria parviflora Lam. (Fumariaceae ) is an annual herbaceous plant that grows in various parts of the Indo-Pakistan subcontinent, Iran, Middle East, and South Asia. It is commonly known as fine-leaf fumitory, Indian fumitory, or wax dolls in English (Orhan et al., 2012). The phytochemical analysis of F. parviflora extract showed the presence of flavonoids, glycosides, tannins, saponins, steroids, phenols, alkaloids, and anthraquinones (Jameel et al., 2014). Pharmacological studies have shown that F. parviflora exhibits hepatoprotective (Alqasoumi et al., 2009), antidiabetic (Fathiazad et al., 2013), anti-inflammatory (Rizvi et al., 2017), antipyretic, analgesic, prokinetic, laxative (Rehman et al., 2012), dermatological, antimicrobial (Jameel et al., 2014), antiparasitic (Al-Shaibani et al., 2009), reproductive, and anticholinesterase activities (Orhan et al., 2004). The present study reports for the first time green synthesis of ZnONPs by using F. parviflora extract and their antibacterial and antioxidant activities.
Materials and methods
Preparation of plant extract
In early April 2019, F. parviflora was obtained from agricultural lands of the village of Benkashkeh in Mamulan District, located 50 km from Khorramabad city, Lorestan province, Iran. The aerial parts of the plant were washed with deionized water, air-dried, and ground into fine pieces.To prepare the extract, 10 g of aerial part powder was heated in 100 ml of deionized water for 15 min at 70°C. The aqueous extract was then filtered using a Whatman No. 1 filter paper and stored at 4°C until used (Jamdagni et al., 2016).
Synthesis of ZnONPs
ZnONPs were synthesized using zinc acetate dehydrate Zn(CH3COO)2 ∙ 2H2O as described previously (Jamdagni et al., 2018). Briefly, 90 ml of zinc acetate solution (0.01 M) was mixed with10 ml of plant extract and incubated overnight at room temperature. Finally, the prepared ZnONPs were separated by centrifugation of the reaction mixture at 4500 rpm for 15 min, followed by washing several times with deionized water and then drying at 80°C for 7–8 h (Gunalan et al., 2012; Salem et al., 2015).
Characterization of NPs
The synthesized ZnONPs were analyzed using a UV-visible spectrophotometer (model number: Unico 1250) in the range of 200–700 nm (Jamdagni et al., 2016). X-ray diffraction (XRD) analysis was performed on an X-ray diffractometer (Philips X-Pert PRO) operated at 40 kV and 40 mA with Cu Kα radiation (1.54060 Å) and at the scanning rate of 2°/min. A sample interval of 0.02° was used in the 2Θ range from 20 to 80° to determine the crystallinity, purity, and size of the NPs (Ambika and Sundrarajan, 2015). The surface morphology of ZnONPs was examined by a scanning electron microscope (SEM) operated at an accelerating voltage at 45 kV (Jamdagni et al., 2016).
Evaluation of the antioxidant activity of ZnONPs with DPPH
The antioxidant activity of ZnONPs was evaluated on the basis of inhibition of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals (Bhakya et al., 2016). One milliliter of ZnONPs at various concentrations (6.25, 12.5, 25, 50, and 100 μg/ml) and ascorbic acid (as a standard) were added to different test tubes. Next, 1 ml of DPPH (1mM) dissolved in methanol was added to each tube and vortexed thoroughly. Lastly, the solution was incubated at room temperature in dark for 30 min. The absorbance at 517 nm wavelength was read with a spectrophotometer, and the percentage of inhibition was calculated using the following equation:
[Azimzadeh, 2009]
where AC is the absorbance of control and AS is the absorbance of sample. Ascorbic acid was used as the standard solution,while 1 mM DPPH was used as a control.
Antibacterial assay
The antibacterial activities of ZnONPs were assessed by disc diffusion and microdilution methods against Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922. Vancomycin and amikacin were used as positive controls.
Disc diffusion method
In sterile conditions, 10 μl of bacterial suspension (opticaldensity (OD) = 0.1 at 600 nm) was inoculated on Muller Hinton Agar (MHA). Next, 30 μl of ZnONPs at different concentrations (25, 50 and 100 μg/ml) were impregnated on discs, and the discs were placed on the inoculated MHA plates and incubated for 24 h at 37°C. The diameters of inhibition zones around the discs were measured and compared with the results obtained for control antibiotics (vancomycin and amikacin). All experiments were performed in triplicates.
Determination of MIC and MBC by using the microdilution method
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of the synthesized ZnONPs were determined by the broth microdilution methodin 96-well micro-plates. A volume of 100 μl of NPs synthesized at the concentration of 100 μg/ml was placed in the first well of the microtiter plate containing 95 μl Mueller Hinton Broth (MHB) medium. Serial dilution was performed by pumping the content of the first well, removing 100 μl from it, and then adding it to the second well; the dilution procedure was thus continued to form a series of dilution from 100 to 0.195 μg/ml. Then, 5 μl of bacterial suspension, equivalent to 0.5 McFarland standard (OD = 0.1 at 600 nm), was added to wells in columns1 to 11. Positive controls (MHB broth with bacterial suspension) and negative controls (MHB broth plus the synthesized NPs without bacterial suspension) were similarly processed. The microplates were incubated at 37°C for 24 h.The absence of bacterial growth was visually checked to define MIC values (Jamdagni et al., 2016). To determine MBC, 10 μl from all wells with no or very little visible bacterial growth was removed and cultured on Mueller Hinton Agar media. The plates were then incubated for 24 h at 37°C. The MBC was defined as the lowest concentration that kills 99.9% of the initial population. All the experiments were repeated three times (Jahanpanahi and Mohamadi Sani, 2016; Nabipour and Dousti, 2018).
Statistical analysis
The experiments were performed in triplicate. Data are expressed as mean ± standard deviation. The data obtained were evaluated by a variance analysis (ANOVA) and Tukey’s test, and P < 0.05 was considered to be statistically significant. The data were interpreted using SPSS software version 16.
Results and discussion
Biosynthesis of ZnONPs
Zinc acetate dihydrate was used to synthesize ZnONPs. The change in the color of acetate after the addition of the extract was the first step indetecting the formation of NPs. During the synthesis of zinc NPs using the F. parviflora extract, a clear change in the color of the mixture from dark brown to light brown indicated the production of ZnONPs (Fig. 1).
Fig. 1
Visual observation of ZnONPs A) aqueous extract of Fumaria parviflora, B) zinc acetate dehydrate, C) plant extract + ZnONPs

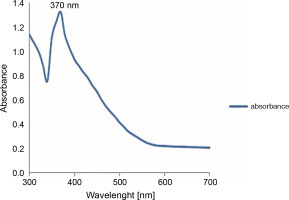
To further confirm the synthesis of zinc NPs, the absorption spectrum of the sample in the UV-VIS spectra was measured. The sample had a sharp absorbance peak at 370 nm, which is in agreement with previous reports (Elumalai and Velmurugan, 2015; Zare et al., 2017), and conforms with the range of light absorption of ZnONPs (360–380 nm) (Nagarajan et al., 2013; Umar et al., 2019) – Figure 2.
XRD analysis
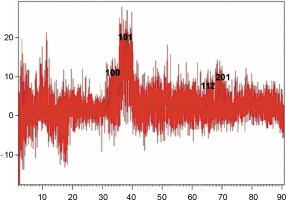
XRD is known as a “fingerprint technique” as it provides a unique characteristic X-ray diffraction pattern of each crystalline solid for its identification. XRD can be used to determine the structure of crystalline solids, i.e., how the atoms are packed together in the crystalline state, what are the interatomic distanceand angle, etc. (Khaing et al., 2018). The XRD pattern of the synthesized ZnONPs clearly indicated the crystalline structure of the synthesized NPs (Fig. 3). The XRD pattern showed four distinct diffraction peaks at 31.86, 36.22, 67.71, and 68.83 degrees. These peaks wereindexed as (100), (101), (112), and (201) diffraction lattice planes, respectively, which confirmed the hexagonal wurtzite structure of the synthesized NPs; this finding is in agreement with Joint Committee on Powder Diffraction Standards (JCPDS) card no. 36–1451 (Talam et al., 2012; Ghamsari et al., 2019; Alamdari et al., 2020). The average size of ZnONPs was calculated from the highest intense peak (101) using the Debye-Scherer equation:
where D is the crystallite size of ZnONPs, λ is the wavelength, β is the full width at half maximum of the diffraction peak and θ is the Bragg angle in degrees (Safawo et al., 2018). The average crystallite size calculated for the synthesized ZnONPs was 16 nm. The particle size of the synthesized ZnONPs was in close agreement with previous findings (Talam et al., 2012; Alwan et al., 2015).
SEM analysis
The morphology and size of the synthesized ZnONPs were determined by SEM analyses (Fig. 4).The ZnONPs were spherical in shape with particle size ranging from 42 to 60 nm, clustered together, and with rough surface of the aggregates. The obtained results were almost identical to the findings of Gul et al. (2019). These authors synthesized ZnONPs by using an aqueous extract of Viola and observed that the NPs were spherical in shape with an average size of 42–60 nm. In another study, the ZnONPs synthesized using an orange fruit peel extract were spherical in shape with an average size of 35–60 nm (Thi et al., 2020). Similar morphologies were also reported in previous studies. Sphericalshaped NPs were reported for those obtainedusing aqueous leaf extracts of three plants, i.e., Aloe vera, Azadirachta indica (L.), Abutilon indicum, and Clerodendrum inerme (Sangeetha et al., 2011; Elumalai and Velmurugan, 2015; Khan et al., 2018).
Antioxidant activity of ZnONPs
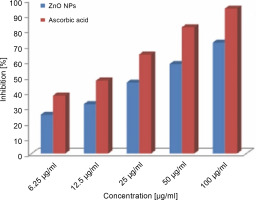
ZnONPs showed effective inhibition activity in the DPPH scavenging assay as compared to the standard ascorbic acid (Fig. 5). The significant antioxidant potential of ZnONPs wasdue to the hydrogen-donating ability of the active phytochemicals of F. parviflora, such as flavonoids, glycosides, tannins, saponins, steroids, phenols, and alkaloids that are capped over ZnONPs (Parashant et al., 2015). The DPPH activity of the ZnONPs was found to increase in a dose-dependent manner. When ZnONPs were added to the DPPH solution, a color change occurred due to the scavenging of DPPH because of donation of a hydrogen atom to stabilize the DPPH molecule, which is responsible for the absorbance at 517 nm (Thomas et al., 2018). Previous studies support the antioxidant activity of ZnONPs (Das et al., 2013; Stan et al., 2016; Safawo et al., 2018). The DPPH values increased in a dose-dependent manner. The recorded value for the lowest concentration of the ZnONPs (6.25 μg/ml) was 25.25 ± 0.04, and this value increased to 72.12 ± 0.12 when the concentration increased to 100 μg/ml. The percent inhibition of DPPH radicals by the ZnONPs was 72.12% at 1000 μg/ml, whereas it was 94.25% for ascorbic acid (standard) at 1000 μg/ml. The radical scavenging capacity of the synthesized ZnONPs was slightly lower than that of the standard ascorbic acid at all the tested concentrations of NPs (Fig. 5). The obtained results also revealed that the recorded IC50 (50% inhibition of free radicals) values of ZnONPs and ascorbic acid (usedas a positive control) for DPPH radical inhibition were 30.86 and 15.13 μg/ml, respectively. This finding suggests that the synthesized ZnONPs showed good antioxidant activity as compared to ascorbic acid. Previously, ZnONPs synthesized by using an aqueous extract of Coccinia abyssinica exhibited the maximum DPPH scavenging activity of 60.12% at 100 μg/ml and an IC50 value of 127.64 μg/ml (Safawo et al., 2018).
Antimicrobial activity of ZnONPs
Disc diffusion method
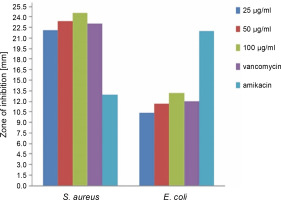
The antibacterial activity of the green synthesized ZnONPs in comparison with vancomycin and amikacin standards was investigated at various concentrations (25, 50, and 100 μg/ml) against S. aureus and E. coli by using the disc diffusion method. The results are shown in Figure 6 and Table 1. It was observed that the synthesized ZnONPs showed anantibacterial effect against S. aureus and E. coli at all the tested concentrations; the synthesized NPs also exhibited enhanced antibacterial activity against S. aureus as compared to the synthetic antibiotics vancomycin and amikacin. The antibacterial activity increased with an increase in the concentration of ZnONPs. This result was consistent with the findings of Sangeetha et al. (2011), where it was found that with the increase in the concentration of ZnO nanoparticles, the growth inhibition also consistently increased. The biosynthesized ZnONPs (at 100 μg/ml concentration) showed the highest zone of inhibition (24.6 mm) against gram-positive S. aureus and the lowest zone of inhibition (13.2 mm) against gram-negative E. coli (P < 0.05), which implies that gram-negative bacteria might show higher resistance to ZnONPs. Our findings were in agreement with other similar reports where the antimicrobial properties of ZnONPs synthesized using different plant extracts were studied. Ambika and Sundrarajan (2015) reported that ZnONPs synthesized using Pelargonium zonale leaf extract had the strongest antibacterial activity against S. aureus (20 mm) than against E. coli (14 mm). On the other hand, Elumalai and Velmurugan (2015) showed a potent antibacterial activity of ZnONPs synthesized using leaf extract of Azadirachta indica (L.), withthe strongest antibacterial activity against S. aureus (19 mm) than against E. coli (17 mm). Sonia et al. (2017) showed strong antimicrobial potential of ZnONPs against both gram-positive and gram-negative bacteria. Some previous studies also showed antibacterial activity of ZnONPs synthesized using different plant extracts against grampositive and gram-negative bacteria (Raghupathi et al., 2011; Sharma et al., 2016; Jahanpanahiand and Ali Mohamadi, 2016; Vahidi et al., 2019).
Microdilution method
The MIC and MBC values of ZnONPs obtained for the pathogenic bacterial species are reported in Table 2. According to these results, the lowest MIC and MBC values of ZnONPs against S. aureus were 1.56 and 3.12 μg/ml, respectively, and the lowest MIC and MBC values against E. coli were 6.25 and 12.5 μg/ml, respectively. The results of MIC and MBC assays were similar to those obtained for the disc diffusion assay. Accordingly, these results indicate that the antibacterial activity of ZnONPs was higher for gram-positive bacteria than for gram-negative bacteria. Our result is also consistent with previous studies. Safawo et al. (2018) reported that S. aureus exhibited higher sensitivity toward ZnONPs with 21 mm zone of inhibition. The MIC value of ZnONPs against S. aureus was 1.25 μg/ml. This could be because gram-positive bacteria lack the outer membrane and have a thick peptidoglycan layer, which makes them sensitive to ZnONPs, unlike gram-negative bacteria that have an outer membrane with a thin peptidoglycan layer rich in lipopolysaccharides, lipids, and lipoproteins that are quite resistant to ZnONPs (Ahmed et al., 2017).
The antimicrobial activities of ZnONPs toward bacteria depend on particle size, powder concentration, morphology, specific surface area,release of reactive oxygen species (ROS), and zinc ions. Generated ROS species, hydrogen peroxide (H2O2), OH– (hydroxyl radicals),
Conclusions
Our study results showed that green synthesis of ZnONPs was conducted successfully from the aqueous extract of F. parviflora. UV-Vis analysis confirmed the synthesis of ZnONPs, and SEM images reported individual particle size in the range of 42–64 nm. Hexagonal structure of the synthesized ZnONPs was confirmed by X-ray diffraction analysis. The DPPH assay indicated that the synthesized ZnONPs have antioxidant activity with IC50 value of 30.86 μg/ml. The synthesized ZnONPs also showed better antibacterial activity against gram-positive bacteria than against gram-negative bacteria.