Introduction
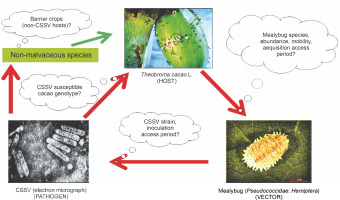
Cacao swollen shoot virus (CSSV) is a devastating pathogen of significant economic importance to the production of cacao (Theobroma cacao L.), especially in West Africa. West African countries account for over 75% of annual global cacao production (Ameyaw, 2019). T. cacao L. is an important tree whose seeds are primarily used for the production of confectionaries such as chocolate (Ploetz, 2016). The cacao industry plays a significant economic role as one of the major non-oil export earners for West African countries (Muller, 2016). However, annual cocoa yield losses attributable to CSSV could range from 50 to 100% in 3 years depending on the severity of outbreak (Rao and Reddy, 2020). Moreover, the accumulation of detailed molecular-based information on the pathogen’s presence in key West African cacao-growing countries has been remarkably slow. The first documented report of CSSV in Nigeria was in 1944 (Murray, 1945), which was followed by a field survey between 1944 and 1949 (Thresh and Tinsley, 1959). This survey aimed to identify and eradicate by cutting symptomatic CSSV-infected cacao trees. Similarly, there have been no new literature available with time on natural vectors of CSSV – mealybugs (Pseudococcidae : Hemiptera ) in Nigeria after Sutherland (1955) report. Of the 25 different mealybug species reported on cacao in West Africa, at least 16 are CSSV vectors (N’Guessan et al., 2014; Obok et al., 2018a; 2018b; Roivainen, 1980). The cacao-CSSV-mealybug interaction complex (Fig. 1) depends on several factors, namely the presence (or absence) of barrier crops, host tolerance (susceptibility and resistance), and the ability of the vector to move (feed) between host plants and to acquire, retain, and effectively transmit the virus from source plants (symptomatic and asymptomatic hosts) to healthy plants (i.e., CSSV-free hosts) (Ploetz, 2007). There is a possibility of missing asymptomatic CSSV-infected cacao trees and to have confusion between nutrient-deficient cacao tress and symptomatic CSSV-infected cacao tress during field surveys (Lockhart and Sachey, 2001). The misidentification of the vector mealybug species might also occur due to morphological variability and complexity of visually observed external taxonomic features of the putative CSSV vector mealybug species (Cox and Freeston, 1985). To address these issues, the use of mitochondrial gene, cytochrome c oxidase subunit I (COI) (Hebert et al., 2003), in DNA barcoding has become an essential tool to compensate classical taxonomy of mealybug species.
Fig. 1
An archetypical disease triangle of cacao-CSSV-mealybug interactions (red arrows show potential mealybug movements and CSSV transmission, and the green arrow indicates potential loss of mealybug infectivity potential and subsequently, CSSV non-transmission)
The importance of the mitochondrial COI gene-based screening (molecular information) over the exclusive use of classical taxonomy (visual morphological features) is based on the mutating rates of mitochondrial DNA in eukaryotes, thus resulting in discernibly wide and narrow genetic variations between and within species, respectively (Kondo et al., 2008). DNA barcoding refers to the procedure of sequencing a short portion of the mitochondrial COI gene from a taxonomically unidentified sample and verifying it against an established collection of barcodes of identified species origin to create a species-level identification (Wilson, 2012). These short fragments of COI are called DNA barcodes. DNA barcodes can significantly advance the identifiation and inventorying of insect-plant associations as they offer a robust documentation of host plant species and validate such trophic associations (Jurado-Rivera et al., 2009). DNA barcoding, therefore, is a rapid, economical, and accurate approach to address problems associated with mis-identification of cryptic species (Hebert et al., 2003; Hebert and Gregory 2005; Jalali et al., 2015) with unresolved morphological matches (Cavalieri et al., 2008; Demontis et al., 2007; Herbert et al., 2016). Hence, the objectives of the present study were to survey, sample and screen for CSSV in Nigerian cacao leaf samples by using an opening reading frame 1 (ORF1) CSSV-specific primer pair in polymerase chain reaction (PCR) and to combine environmental scanning electron microscopy (ESEM), histology and PCR COI-based DNA barcoding to characterise the CSSV vector mealybugs species found on cacao in Nigeria.
Materials and methods
Sample collection
Field surveys of cacao-producing sites were conducted in Abia, Akwa Ibom, Cross River, Edo, Ondo and Oyo States from 1 June to 31 August 2012, 1 February to 30 April 2013 and 1 May to 31 July 2016. Whole symptomatic and nearby asymptomatic cacao leaves (Fig. 2A) from tree canopies were collected. All samples were collected from trees at a distance of 10 m apart. GPS locations (latitude and longitude) of every sampled tree were acquired with a Garmin eTrex Venture HC navigator (Garmin International Inc., Kansas, USA). The sampled cacao leaves were freed of all dirt and debris and then flat placed inside Ziploc® bags (S. C. Johnson, Wisconsin, USA).
Fig. 2
A) Adaxial surface of symptomatic cacao leaf infected with CSSV; B and C) mealybug infestation on stems and D–G) pods

By using a fine paint brush (#2), live samples of adult female mealybugs were carefully collected from trunks/stems, leaves and pods of cacao trees (Fig. 2D–2G). It was necessary to gently tap the mealybug and allow an interval of 1–2 min in order to ensure that they withdraw their stylets from plant tissues, if in feeding position.
Where the mealybugs occurred either in colonies or individually, one sample was separately placed inside a 1.5 ml Eppendorf® tube. All samples were stored at 4°C, and phytosanitary certificates were later obtained within 36 h before the samples were couriered to the University of Reading, United Kingdom, for onward storage at 4°C pending further analysis.
Genomic DNA extraction and PCR: cacao
Approximately 80–100 mg excised cacao leaf discs were placed in a 2 ml Eppendorf® tube with one 5 mm stainless steel bead, secured with lids, snap frozen with liquid nitrogen (at –196°C) and crushed to fine powder by using the TissueLyserII (Qiagen, Hilden, Germany). Genomic DNA was extracted from each leaf sample using the DNeasy Plant Mini Kit according to the manufacturer’s protocol (Qiagen). Quantification and assessment of purity of the genomic DNA concentration were performed using a NanoDropTM 2000 spectrophotometer (Thermo Fisher Scientific Inc., Abingdon, UK). An ORF1 primer pair (Table 1) was used for PCR-based CSSV screening. The primer pair was designed in the conserved regions of the six published sequences of the CSSV genome (National Centre for Biotechnology Information (NCBI) accession number AJ608931) (Hagen et al., 1993; Muller and Sackey, 2005). Each PCR reaction mixture (25 μl) contained 2 μl genomic DNA, 2.5 μl ORF1 primer pair (2 μM), ddH2O and 12.5 μl BioMix (Taq polymerase and oligonucleotides) (Bioline). All PCR reactions were performed in a G-STORM GS2 thermal cycler (Gene Technologies Ltd., Essex, UK) under the following conditions: initial denaturation for Taq activation at 94°C for 4 min, then 40 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, elongation at 72°C for 2 min, with a final elongation at 72°C for 5 min and storage at 10°C for 30 s.
Table 1
CSSV-specific PCR primer pairs used for screening cacao leaves
| Name | Orientation | Sequence (5ˊ – 3ˊ) |
|---|---|---|
| ORF1 | forward | AGTATCCARGARTGGTAYGA |
| reverse | TCATTGACCACCCAYTGRTC |
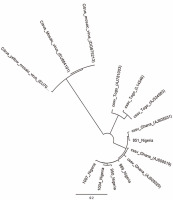
The PCR products were analysed in 1% agarose gel electrophoresis and stained with 3 μl ethidium bromide (from a stock solution of 10 mg/ml). The loading dye (2 μl) was added to 8 μl of the PCR product. For sizing the DNA in the PCR products, 2 μl of Bioline Hyper-LadderTM 1000 bp was used. Electrophoretic gels were set to run at 110 V for approximately 1 h with Power-PacTM Basic. The images were viewed under a UV light source in a closed chamber with GelDoc-ItTS2 Imager run with UVP TS2 Software (Ultra-Violet Products Ltd., Cambridge, UK). The corresponding PCR products were purified with the NucleoFast® DNA, RNA and protein purification kits (Macherey-Nagel GmbH and Co., Düren, Germany) according to the manufacturer’s protocols. Source BioScience Ltd. (Oxford, UK) conducted direct DNA sequencing. A phylogenetic tree of CSSV sequences (neighbour-joining method) using the Tamura-Nei model was generated using Geneious software version 9.0 (Auckland, New Zealand) for comparison with Citrus yellow mosaic virus (CYMV) badnavirus outgroup.
Mealybug microscopy
Prior to microscopy, histology and genomic DNA extraction, the sampled female mealybugs were prepared according to the protocols of Banks and Williams (1972). For ESEM, whole body female mealybug samples were placed inside a 2 ml Eppendorf tube with 0.5 ml 2% DECON 90 surfactant (DECON Laboratories Ltd., East Essex, UK) and incubated at room temperature for 24 h. Next, up to 0.4 ml of the surfactant was carefully pipetted out, and 0.5 ml of ddH2O was added to completely and carefully loosen and rinse off any waxy covering left on the surfaces of mealybugs. The wax-free mealybug samples were carefully dislodged and left to dry on a secured Whatman® 3MM blotting paper for 30–60 s. The dried samples were subsequently prepared according to Sirisena et al. (2015) for ESEM using a Field Emission Inc. (FEI) Quanta 600F (FEI UK Ltd., Cambridge, UK) equipment. ESEM images of uncoated whole-body samples were captured under low vacuum, constant pressure (0.68 Torr) and high voltage (5.0–12.5 kV). For finer and intact morphological structures, the samples were gold-coated and captured under high vacuum at 20 kV.
Mealybug histology
Histological slides of individual mealybug samples were prepared according to the protocols of Ben-Dov and Hodgson (1997), European and Mediterranean Plant Protection Organization (2005), Watson and Chandler (2000) and Williams and Granara de Willink (1992). By using a stereo microscope, a dorsolateral incision was made on each of the mealybug samples with a fine-tip stainless steel needle. This enabled easy maceration and gentle expulsion of internal tissues, gut and eggs (for gravid females) from individual mealybug samples. These contents were pipetted and stored in new Eppendorf® tubes for subsequent genomic DNA extraction. Slides were later prepared from the mealybug exoskeleton left behind. The fixed slides were viewed and photographed under a Leica Z6APO/DFC450 digital microscope camera with a Leica LAS Live Image Builder (Leica Microsystems Ltd., Milton Keynes, UK).
Photographed histological slides of the adult female mealybug specimens and ESEM images obtained were used for morphological identification of the mealybug samples based on the established keys developed to distinguish female mealybug species of the family Pseudococcidae (Cox, 1989; Cox and Freeston, 1985; Miller et al., 2014; Williams and Watson, 1988). The morphological identification was based on a combination of features and structures from each of the examined mealybug species (slides and ESEM), including and not limited to the number of setae, type and number of pores, absence of denticle on the claws, number of body segments, and number of segments on the antennae.
Mealybug genomic DNA extraction and PCR
Mealybug genomic DNA extraction was performed using the DNeasy Blood and Tissue kits (Qiagen) according to the manufacturer’s protocol. For each of the extracted genomic DNA sample, a 25 μl PCR reaction mixture was prepared. In the negative control sample, ddH2O was added instead of the DNA template. Primers were designed from the available pseudococcid sequences to amplify a 379-bp partial region of the COI gene (Wetten et al., 2016). Each PCR reaction mixture contained 2 μl genomic DNA, 2.5 μl COI forward primer (2 μM) (Table 2), 8 μl ddH2O, and 12.5 μl BioMix (Bioline). All PCR reactions were performed in a G-STORM GS2 thermal cycler (Gene Technologies Ltd.). The first step (5 cycles) included initial DNA denaturation for Taq activation at 94°C for 1 min, annealing at 46°C for 1 min 30 s, and elongation at 72°C for 1 min 30 s. The second step (35 cycles) included denaturation at 94°C for 1 min, annealing at 50°C for 1 min 30 s, and elongation at 72°C for 1 min, with a final elongation at 72°C for 5 min and storage at 10°C for 30 s. The PCR products were assessed by 1% agarose gel electrophoresis (as described above). The PCR products were Sanger sequenced on both strands by Source BioScience Ltd.. Consensus sequences were produced, and alignments were manually edited with Geneious 9.0.
Results
CSSV detection in Nigerian cacao: presence and spread
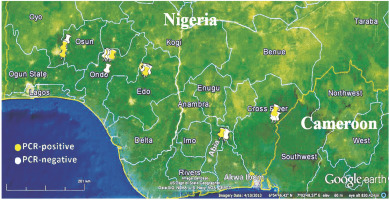
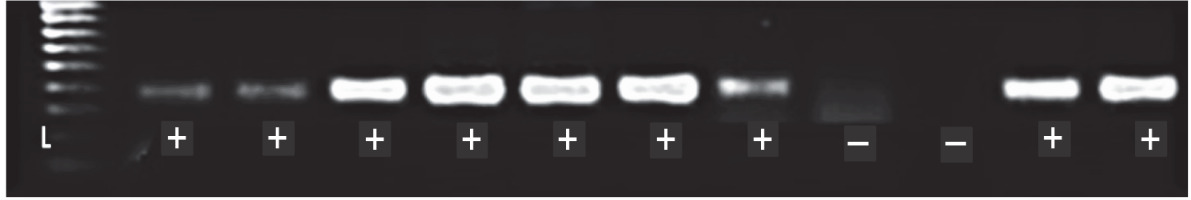
A total of 1052 cacao trees were inspected for CSSV symptoms across the six major cacao-growing areas in Nigeria. Symptomatic leaves of CSSV-infected trees were characterised by leaf chlorosis, interveinal chlorosis with mottling and swollen shoots in very severe cases. The highest number of trees inspected were in Oyo State (234), while Edo State recorded the least number of trees inspected (94). Overall, the number of symptomatic leaves collected was 477, while the number of collected asymptomatic leaves was 2091. Oyo State had the highest number of symptomatic (132) and asymptomatic (439) cacao leaf samples, while Edo State had the least number of symptomatic (39) and asymptomatic (185) cacao leaf samples. To detect CSSV, genomic DNA was extracted from the sampled cacao leaves, and the average genomic DNA concentration ranged from 5 to 50 ng/μl. From the 2568 cacao leaf samples (i.e., 477 symptomatic and 2091 asymptomatic cacao leaves) (Table 3) collected for CSSV screening with an ORF1 primer using PCR, the virus was detected in 166 leaves (Table 4). CSSV was detected in 124 of 477 symptomatic cacao leaves sampled, while 42 of 2091 asymptomatic cacao leaves tested positive for CSSV. The PCR-amplified gene sequences were sequenced from symptomatic and asymptomatic leaves and compared with the data from the NCBI database. The highest number of PCR-positive CSSV-infected symptomatic and asymptomatic cacao leaf samples was found in Ondo State (42), while the lowest CSSV PCR detection level was observed in Edo State (11). Previous studies have reported the presence of CSSV in farms of Ondo, Oyo and Cross River States, Nigeria. In the present study, we reported for the first time the presence of CSSV in new sites, namely Abia, Akwa Ibom and Edo States. All CSSV-positive PCR products from CSSV-infected cacao leaves yielded expected DNA fragment sizes of 410 bp as observed in 1% agarose gel electrophoresis. Representative DNA sequences of the PCR-positive cacao leaf samples from each of the locations (Fig. 3) were analysed, consensus sequences were generated and alignments were manually edited. Genetic similarity and relatedness were determined using the neighbour-joining method as described by Wetten et al. (2016). Our results showed that DNA sequences of CSSV detected from cacao leaves in Nigeria were distinct from those from Togo, but closely related to CSSV sequences from Ghana deposited in the NCBI database (Fig. 4). In summary, PCR-based screening with the ORF1 primer revealed CSSV presence in Ondo, Oyo, Cross River, Abia, Akwa Ibom and Edo States (Fig. 5), with the percentage of CSSV detection ranging from 6.6 to 31.9% and the prevalence rate ranging from 33.3 to 83.3% (Table 4).
Table 3
Mealybug and cacao leaf collections for DNA barcoding and CSSV screening
| Location (state) | Trees inspected | Mealybugs collected | Cacao leaves collected | |||||
|---|---|---|---|---|---|---|---|---|
| leaf | pod | trunk (stem) | total | symptomatic † | asymptomatic | total | ||
| Oyo | 234 | 103 | 46 | 19 | 168 | 132 | 439 | 571 |
| Ondo | 218 | 69 | 42 | 17 | 128 | 109 | 423 | 532 |
| Cross River | 214 | 53 | 27 | 14 | 94 | 103 | 419 | 522 |
| Abia | 174 | 24 | 16 | 4 | 44 | 51 | 380 | 431 |
| Akwa Ibom | 118 | 18 | 11 | 8 | 37 | 43 | 245 | 288 |
| Edo | 94 | 28 | 14 | 13 | 55 | 39 | 185 | 224 |
| Total | 1052 | 295 | 156 | 75 | 526 | 477 | 2091 | 2568 |
Table 4
CSSV detection in cacao in six major cacao growing areas in Nigeria
| Location (state) | PCR-positive cacao leaf samples | ||||||
|---|---|---|---|---|---|---|---|
| symptomatic † | asymptomatic | total | detection [%] | number of areas surveyed | number of infected areas ** | prevalence [%] †† | |
| Ondo | 41 | 12 | 53 | 31.9 | 6 | 5 | 83.3 |
| Oyo | 34 | 8 | 42 | 25.4 | 8 | 6 | 75.0 |
| Cross River | 16 | 10 | 26 | 15.7 | 8 | 4 | 50.0 |
| Abia | 13 | 6 | 19 | 11.4 | 3 | 1 | 33.3 |
| Akwa Ibom | 11 | 4 | 15 | 9.0 | 6 | 2 | 33.3 |
| Edo | 9 | 2 | 11 | 6.6 | 3 | 1 | 33.3 |
| Total | 124 | 42 | 166 | 100 | 34 | 19 | na |
† based on visual symptoms of an archetypical CSSV infected cacao plant which include leaf chlorosis, interveinal chlorosis with mottling and swollen shoots in very severe cases;
Fig. 3
DNA fragments of CSSV PCR products with expected size (410 bp); L – HyperLadderTM 100 bp (Bioline, UK), (+/–) – PCR positive/negative
Morphological identity of the sampled mealybugs in Nigerian cacao
A total of 1052 young and old cacao trees were inspected (up to breast height) for the presence of mealybugs (Hemiptera: Pseudococcidae). Samples were collected from leaves (usually under the leaf surface) (295), cacao pods (156) and trunks (stems) (75) (Table 3). Compared to other locations, the number of mealybugs on cacao leaves, pods and trunks was higher in Oyo, Ondo and Cross River States. The identity of 526 mealybug samples was determined by visual observation and confirmed by an entomologist, Dr. Collins Campbell (University of Reading, UK). This provided baseline information for identifying the mealybug samples (Hemiptera : Pseudococcidae ). The highest number of female mealybugs (juveniles and adults) were collected in Oyo State (168), while the smallest number was recorded in Akwa Ibom State (37). Morphological images of the whole bodies of the sampled female mealybugs that were slide mounted and acquired under ESEM are shown in Figures 6 and 7. Samples with distinct morphological features (head, eye, denticle on claw, setae, cerarii, antennae, and pores) were grouped accordingly. Prepared slides of the sampled mealybug set and imaged are presented in Figures 8A–8D. Vouchers were prepared for mealybug slides that had matching morphological features using an online interactive tool (http://idtools.org/id/scales/key.php?key=mealybugs) developed by Miller et al. (2014) for the identification of scale insects to the species level. The tool is used to morphologically identify hemipteran species of the family Pseudococcidae globally, including both temperate and tropical mealybug species. Analysis based on morphological keys postulated for species identification of mealybug as described by Ezzat and McConnell (1956) and Miller et al. (2014) were used to classify the collected mealybug samples to the species level. Consequently, each mealybug sample identified was grouped into seven (7) distinctive groups as summarized in Table 5. Significant morphological variations were observed, including the number of cerarii pairs found on the whole body, number of coni on each seta and number of antenna segments on each antenna. No claw denticles (small-tooth) were observed in any groups, thus implying that all morphologically identified mealybugs belonged to the family Pseudococcidae.
Table 5
Occurrence and abundance of mealybugs (Hemiptera : Pseudococcidae n) in six major cacao growing areas in Nigeria †
| Mealybug species (nearest NCBI CO1 match) | Location (State) | Total | Abundance [%] | |||||
|---|---|---|---|---|---|---|---|---|
| AB | AK | CR | ED | ON | OY | |||
| Planococcus citri (Risso) | 24 (54.5) | 18 (48.6) | 46 (48.9) | 28 (50.9) | 84 (65.6) | 97 (57.7) | 297 | 56.46 |
| Formicococcus njalensis (Laing) | 9 (20.5) | 11 (29.7) | 17 (18.1) | 16 (29.1) | 23 (18.0) | 42 (25.0) | 118 | 22.43 |
| Planococcus minor (Maskell) | 5 (11.4) | 3 (8.1) | 8 (8.5) | 6 (10.9) | 9 (7.0) | 12 (7.1) | 43 | 8.17 |
| Paracoccus marginatus (Williams & Granara de Willink) | 1 (2.3) | 1 (2.7) | 3 (3.2) | 1 (1.8) | 3 (2.3) | 6 (3.6) | 15 | 2.85 |
| Pseudococcus jackbeardsleyi (Gimpel & Miller) | 0 (0.0) | 0 (0.0) | 13 (13.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 | 2.47 |
| Ferrisia virgata (Cockerell) | 2 (4.5) | 2 (5.4) | 2 (2.1) | 1 (1.8) | 2 (1.6) | 3 (1.8) | 12 | 2.28 |
| Paraputo loranthi (Strickland) | 1 (2.3) | 1 (2.7) | 2 (2.1) | 1 (1.8) | 3 (2.3) | 3 (1.8) | 11 | 2.09 |
| Unidentified species †† | 2 (4.5) | 1 (2.7) | 3 (3.2) | 2 (3.6) | 4 (3.1) | 5 (3.0) | 17 | 3.23 |
† based on combined morphological and molecular identification; mealybug samples were collected from all the areas (in brackets) in each of the locations (states) namely AB – Abia (Umuahia, Bende, Ibeku), AK – Akwa Ibom (Ebo, Ikpe Ikot-Nkon, Ebam-Ikot, Okpoto, Itu-Mbonuso and Iwere), CR – Cross River (Akamkpa, Boki, Etung, Ikom, Ajassor, Obanliku, Obubra, Obudu), ED – Edo (Uhonmora, Ekpoma, Irrua), ON – Ondo (Owena, Idori, Alade, Gberiwojo, Aponmu, Wasimi), OY – Oyo (Akanran, Offa-Igbo, Koroboto, Kate, Ona-Ara, Lagelu, Ido, Akinyele);
Fig. 6
Low vacuum ESEM of whole adult female mealybug sample showing A) body segments; B) lateral filaments (lf), cerarii, three pairs of legs, circulus (cr) (3rd/4th abdominal segments); C–D) head and eye (ey) with preocular (po) and ocular (o) cerarii and antenna (an); E) coxa (cx), femur (fm) tibia (ti) and tarsus (tr); F) wax (w) from trilocular pores
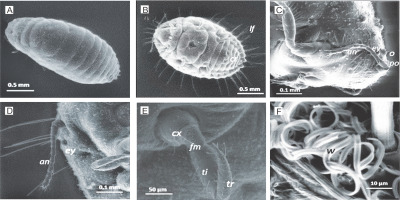
Fig. 7
High vacuum gold-coated SEM images of adult female mealybug showing A) claw (cl), claw digitules (cd) and tarsal digitules (td); B) conical setae (cs); C) trilocular pores (tp) and conical setae (cs) on the anal lobe
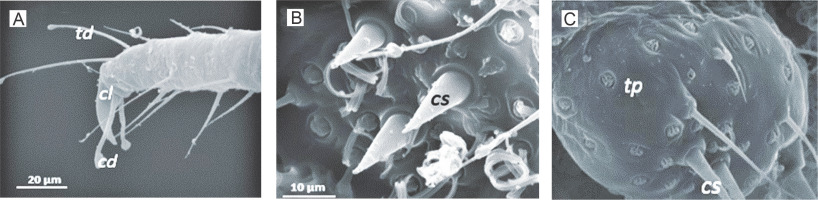
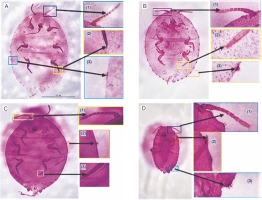
Fig. 8
A) Whole-body slide with 18–19 pairs of cerarii showing (1) 8–9 segmented antennae, (2) a claw with no denticle and (3) setae with three coni; B) whole-body slide with 17 pairs of cerarii (1) 8 segmented antenna, (2) setae with three coni and (3) claws with no denticles; C) whole-body slide with 18 pairs of cerarii showing (1) presence of 8 segmented antennae, (2) setae with three coni and (3) claw with no denticles; D) whole-body slide with 17 pairs of cerarii showing (1) 8–9 segmented antenna, (2) claw with no denticles and (3) coni with 3–4 setae
Our results showed that the mealybugs Ferrisia virgata, Formicococcus njalensis, Planococcus citri, Planococcus minor and Pseudococcus jackbeardsleyi were identified in the current study. None of the mealybugs had denticles on claws, but cerarii were present. Only Planococcus citri and Planococcus minor had antennas with 7 segments. Ferrisia virgata, Formicococcus njalensis, Paracoccus marginatus and Pseudococcus jackbeardsleyi had antennas with 8 segments. However, none of the samples had antenna segments that were either less than 7 segments or greater than 8 segments. Only Ferrisia virgata had two pairs of cerarri on each side of the body, while Paracoccus marginatus was the only sample to have 9 to 16 pairs of cerarri. Remarkably, samples that had 17 pairs of cerarri were either Pseudococcus jackbeardsleyi or Paracoccus marginatus. Both species had an oral-rim tubular duct and triocular pores, which were absent in other identified samples. Albeit, it was not convincing enough to identify these two species based on only these features.
Molecular identity and abundance of sampled mealybugs on cacao in Nigeria
Body content of mealybug samples used for histology and microscopy was extracted, coded and stored at –80°C for subsequent genomic DNA extraction and quantification. The extracted average genomic DNA concentration ranged from 60 to 100 ng/μl (from whole female adult/juvenile mealybugs). DNA barcodes of the morphologically characterised mealybug samples were generated after performing COI-based PCRs, and the products were separated by electrophoresis in 1% agarose gels to verify the DNA quality (Fig. 9.) The extracted genomic DNAs and purified PCR products allowed genetic characterization and identification of each COI PCR-positive mealybug sample. The results (Fig. 10) showed COI DNA matches based on the nearest published COI sequence, except for the unidentified samples. Comparisons were made using a selection of NCBI reference samples. The morphology of individual mealybug samples determined by scanning microscopy and histology correlated with their COI sequences. By using the combination of histological and classical taxonomy keys for hemipterans (Pseudococcidae) and COI-based DNA barcoding, mealybug species that were identified in the present study from the six major cacao-growing regions were as follows: Ferrisia virgata (Cockerell), Formicococcus njalensis (Laing), Paracoccus marginatus (Williams and Granara de Willink), Paraputo loranthi (Strickland), Planococcus citri (Risso), Planococcus minor (Maskell) and Pseudococcus jackbeardsleyi (Gimpel and Miller). Based on the results of DNA matches, the following species were identified: 297 Planococcus citri, 118 Formicococcus njalensis, 43 Planococcus minor, 15 Paracoccus marginatus, 13 Pseudococcus jackbeardsleyi, 12 Ferrisia virgata and 11 Paraputo loranthi. However, there were 17 samples with COI sequences that did not match any of the Pseudococcid sequences amplified from a partial region of the CO1 gene. In terms of ranking, the six major cacao-growing areas (States) in Nigeria showed the following order with regard to the total number of mealybugs obtained from cacao: Oyo > Ondo > Cross River > Edo > Abia > Akwa Ibom.
Fig. 9
Nigerian mealybug post-SEM COI PCR images showing the expected 398-bp DNA fragments (L – HyperLadderTM 100 bp (Bioline, UK), (+) – PCR positive, (‒) – PCR negative (non-mealybug), O – negative control (water, no DNA) and j – positive control (DNA of a known mealybug)

Fig. 10
Cytochrome c oxidase phylogeny (neighbour-joining method) of mealybug samples (with M prefixes) collected from Nigerian cacao; the Planococcus citri and Planococcus minor clades are magnified and highlighted in blue in comparison with reference samples (AB, AF, EU, GQ and JN prefixes)
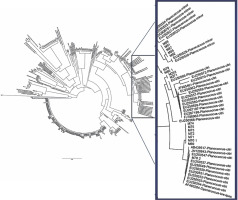
The phylogenetic relationship between the mealybug species characterised in Nigeria and across other cacao-growing countries showed that most of the samples were of the genus Planococcus (Fig. 10). The present study also showed that the genus Planococcus (Planococcus citri and Planococcus minor) had the highest abundance of 64.63%, followed by Formicococcus njalensis with 22.43% abundance (Table 5). Overall, the genus Planococcus was the most represented in the collection of mealybugs from Nigeria. Phylogenetic grouping of each sample against those already submitted to NCBI Gen-Bank enabled the identification of the sampled species. However, where there were no similarities of samples with NCBI reference samples, these were grouped as “unidentified” species (Table 5). The abundance of all other identified and unidentified species ranged from 2.09 to 3.23%. Among the mealybug samples identified was Pseudococcus jackbeardsleyi Gimpel and Miller (Hemiptera: Pseudococcidae), which is a rare species of mealybugs and has been reported only in West Africa in a CSSV-infected farm in Buyo, Côte d’Ivoire by N’Guessan et al. (2014). The ease of identifying this species from other African Pseudococcus species is associated with the presence of sclerotised rims around the eyes containing simple pores. This species was reported only in Ajassor and Etung cacao farms in Cross River State, and its overall abundance was 2.47%. CSSV acquisition, retention and transmission abilities of Pseudococcus jackbeardsleyi are yet to be examined despite sharing the same genus with a known CSSV vector mealybug species, Pseudococcus longispinus (Targioni Tozzetti).
Discussion
The presence of the CSSV in West Africa was reported already over nine decades ago (Muller, 2016). Earlier efforts were directed to establish its genetic details (Hagen et al. 1993; Lot et al. 1991), detection (Muller et al. 2001), molecular variability (Muller and Sackey, 2005), presence, spread and genetic diversity in West African cacao-growing countries (Chingandu et al., 2017; Dzahini-Obiatey et al., 2010; Kouakou et al., 2012; Oro et al., 2012a; Oro et al., 2012b; Muller et al., 2018) as well as CSSV acquisition, retention, transmission and feeding pattern of vector and putative vector mealybug species on cacao (Obok, 2015; Obok et al., 2018b). There has been paucity of information on the spread and molecular diversity of CSSV and its mealybug vectors in Nigeria. However, in the cacao-producing areas surveyed in the present study, the presence of the virus was evident as most of the cacao farms, locations and sites sampled with symptomatic cacao leaves were characterised with various symptoms of the viral disease, such as chlorosis of young and mature leaves, malformation of pods, swollen stems and die-back (Fig. 2). Although visual symptoms are inadequate in signifying viral genomic associations of CSSV, these visual observations were in consonant with archetypal CSSV infection symptoms on cacao as presented by Muller (2016).
Previous reports of CSSV presence in Nigeria based on visual observations (symptoms), without molecular evidence, indicated that the virus was present in Nigeria only in the then western providences of the country (Lister and Thresh, 1957) where cacao production has been on-going to date (now majorly Oyo and Ondo States). Later, by using enzyme-linked immunosorbent assay (ELISA), symptomatic cacao leaves were screened for CSSV and reported in Ibadan and Oyo States (Dongo and Orisajo, 2007). The occurrence of CSSV in Nigerian cacao was first reported in 1944, and the virus was present in the Western parts of the country in Egbeda, Abaku, Olanla, Offa-Igbo, Balogun, Ife, Ibadan, Ilaro, Araromi and Ikire States (Obok, 2015). The diseased cacao trees exhibited temporary, slight and severe symptoms, which were attributed to the differences existing among symptomatically distinct isolates of CSSV reported in Nigeria (Thresh, 1961). The isolates were named after their localities. The results of the PCR analysis showed that CSSV was detectable in field-collected cacao leaf samples in previously unreported cacao-growing areas, namely Abia. Akwa Ibom and Edo States. The affected areas have not been previously characterised as regions of CSSV presence in Nigeria.
Mealybug species can exhibit diverse body features under several situations; therefore, assessment based on body shape may not be a perfect yardstick to identify species in mealybugs (Cox, 1989; Cox and Freeston, 1985; Cox and Pearce 1983; Kaydan et al., 2015). Variation in morphological features were also observed within the identified groups (Figs. 6–8). This ranged from an unequal number of antenna segments on each antenna with no apparent loss of segment due to the preparation protocol. Mealybug species (members of same group(s)) with similar morphological features were also identified with comparable COI gene sequences. In the present study, the exact identities and genetic relationships of theses mealybugs were closely matched with the published Pseudococcid sequences deposited in the NCBI database. In previous studies, where a large number of morphological characteristics were used for the identification process for mealybugs, discriminant function analyses (DFA) and principal component analyses (PCA) were used, and these analyses were barely useful because of rounding up effects (Padi and Hollander, 1996). A typical example is possible misidentification of Planococcus citri as variants, Planococcus sp. nr. minor and Planococcus sp. A, based on morphological discrimination using DFA and PCA (Padi, 1995). This error was not uncommon because of lack of molecular tools to aid characterisation of cryptic mealybug species with which coinciding morphological characteristics often exist. Interestingly, in some cases, Planococcus citri and Planococcus minor were found to have antennas with 8 segments – a confusion that has been reported to cause misidentification of these two species based on morphological features.
The inherent limitations associated with the use of visual observations in the identification of similarly appearing mealybug species (e.g., Planococcus citri and Planococcus minor) in the present study was resolved with the robust discriminatory power of DNA barcoding, and this was in concordance with the findings of Abd-Rabou et al. (2012) and Rung et al. (2008). The use of COI-based DNA barcode assay owes its superiority to the rapid mutation rate of the COI gene coupled with its highly conserved genetic integrity that allows for the discrimination of closely related species (Park et al., 2010; Obok, 2015). COI is a functional gene in the cellular respiratory activity of species (Wilson et al., 2019), which allows for identification match of each individual species. In the present study, COI results showed that mealybug species were distinctly identified and revealed exact phylogenetic relationship within and between species (Table 5 and Fig. 10). The confirmation of the presence of non-indigenous mealybug species in new locations within the cacao-growing areas in Nigeria was an indication of further potential spread of CSSV to new cacao sites.
The identification of Pseudococcus jackbeardsleyi (Hemiptera : Pseudococcidae ) in Ekimayan Village, Etung Local Government Area, Cross River was its first report in Nigeria and second to be reported in West Africa after its first report in Buyo, which was an area infected with CSSV in Cote d’Ivoire 2013 (N’Guessan et al., 2014); this implied that Pseudococcus jackbeardsleyi could be a very important vector of CSSV in West Africa. Although each identified mealybug species could vary in their efficiency in acquisition, retention and transmission of CSSV between infected and non-infected host plants (Obok et al., 2018b; Roivainen, 1980; Roivainen, 1976), the presence of these mealybug species (vectors and putative vectors of CSSV) across cacao farms in the east and south of Nigeria further suggest that CSSV could be spread wider in Nigeria than earlier reported to be found only in the western part of the country.
Results reported by Strickland (1951a,1951b) on the relative abundance of mealybug species within the contemporary Nigerian cacao crop highlighted the dominance of Planococcus citri in Nigeria. Bigger (1981) also examined mealybug diversity around Tafo in Ghana from 1971 to 1976 and found that Formicococcus njalensis, occurred at the highest density on cacao trees, followed by Planococcus citri which had a higher probability of occurrence over Formicococcus njalensis. However, it could be argued that earlier studies on mealybug abundance were inconclusive as conflicting findings on the morphological identity of mealybug species were commonly reported in the literature (Cox, 1989; McKenzie, 1967; Thorold, 1975).
Conclusion
Re-emergence of CSSV was reported in areas that have previously experienced CSSV outbreaks (prevalence), namely Cross River, Ondo and Oyo States. New CSSV outbreaks were reported in Abia, Akwa Ibom and Edo States. In previous reports, decades after the establishment of CSSV presence in Nigeria, the infection was found only in the Western part of Nigeria. Results of the present study show that CSSV is present in all the major cacao-growing areas in Nigeria. This study has provided valuable molecular-based data on the presence, spread, re-emergence and new outbreaks of CSSV in major cacao-growing areas in Nigeria by using an ORF1 CSSV-specific primer PCR screening approach. In addition to these data, complementing information on the identity of several mealybug species (vectors and putative vectors of CSSV) using COI-based DNA barcoding has been provided. In addition to six of the predominant mealybug species identified in all the six major cacaogrowing areas, a new mealybug species, Pseudococcus jackbeardsleyi, was reported only in Cross River State, one of the major cacao-growing areas in the southern part of Nigeria. Pseudococcus jackbeardsleyi is a mealybug species in the genus that has other vectors of CSSV e.g., Pseudococcus longispinus . The importance of understudying Pseudococcus jackbeardsleyi as a putative vector in the transmission of CSSV cannot be overemphasised. In terms of the frequency of occurrence, the identified mealybug species followed the order: Planococcus citri (Risso) > Formicococcus njalensis (Laing) > Planococcus minor (Maskell) > Paracoccus marginatus (Williams and Granara de Willink) > Pseudococcus jackbeardsleyi (Gimpel and Miller) > Ferrisia virgata (Cockerell) > Paraputo loranthi (Strickland).
Expanded area surveys and in-depth molecular analysis must therefore be established in both old and new areas experiencing CSSV outbreaks in Nigeria. To establish whether the infection rate increases annually, annual routine farm surveys, diagnostics, and reports should not be neglected. It is also recommended that stringent and adequate screening measures should be implemented to restrict the spread of plant materials from areas of known CSSV incidence to new areas. For future studies, a larger number of mealybug species should be characterised, as the use of COI-based DNA barcoding in combination with morphological identification of mealybugs proves to be a cost-efficient and reliable option to adopt. Dependable taxonomic information, once provided, could guide in using appropriate generic- and/or species-specific management strategies with the aim of controlling the spread of CSSV by vector mealybug species. There is therefore a great need to update and continuously accumulate information on CSSV and its mealybug vectors in cacao-growing areas in Nigeria.