Introduction
Scutellaria is the second largest genus within the Lamiaceae family with over 360 worldwide species (Ranjbar and Mahmoudi, 2017). Scutellaria araxensis Grossh is a perennial herb native to Iran and parts of Azerbaijan (Ranjbar and Mahmoudi, 2017). It is also known as Araksinsky skullcap and is used in folk medicine to treat several ailments (Gharari et al., 2020a). S. araxensis often grows in Marl lands at an altitude of 800 m with an average annual rainfall of 200 to 270 mm.
The main components of the volatile oil from the aerial parts of S. araxensis are oxygenated monoterpenes with cis- anethole (28.5%) and p-menthan-3-one as the principal components (Gharari et al., 2020a). The essential oil of S. araxensis shows in vitro antimicrobial activity against some gram-positive and gram-negative bacteria such as Staphylococcus aureus and Escherichia coli (Gharari et al., 2020a). S. araxensis contains a wide variety of flavones, especially avonoids, such as flavone aglycones (baicalein and wogonin), glycosides (baicalin and wogonoside), and methylated derivatives (Gharari et al., 2020b). In addition, by using HPLC-DAD-ESI/MSn analysis, four known phenylethanoid glycosides, namely martynoside, acteoside, allysonoside, and verbascoside, were identified from the root and shoot parts of in vitro cultured S. araxensis (Gharari et al., 2020b). Recent pharmacological research confirmed that the Scutellaria genus and its isolated pure compounds possess beneficial bioactivities such as antitumor, anti-inflammatory, hepatoprotective, antibacterial, anticonvulsant, and antiviral activities (Shang et al., 2010; Gharari et al., 2021a).
The commercial use of herbal medicines has led to the danger of extinction of many wild medicinal herbs. The Forests, Rangeland and Watershed Management Organization of Iran has classified approximately 2300 plant species with significant pharmaceutical effects that are used in Iran traditional medicine (Jafari et al., 2019). Considering the pharmaceutical properties of S. araxensis and the low rate of its distribution in natural habitats, it has become necessary to develop efficient alternative tools for the propagation and conservation of S. araxensis genotypes. In vitro conservation is one of the plant tissue culture techniques that is widely used for the rapid and efficient propagation of several endangered and endemic medicinal species under sterile conditions (Debnath et al., 2006). In recent years, intensive efforts have been made to conserve and propagate several endangered medicinal plants such as Paraisometrum mileense and Tuberaria major by in vitro conservation techniques from a small quantity of the explant sources without any notable effects on wild-type populations (Coelho et al., 2014; Lin et al., 2014; Monemi et al., 2014). On the basis of these facts, the present investigation aimed to evaluate the effect of 6-benzylaminopurine (BAP), thidiazuron (TDZ), α-naphthalene acetic acid (NAA), and indole-3-butyric acid (IBA), either alone or in combination, on callus induction, shoot organogenesis, and multiplication of in vitro cultured S. araxensis leaf, petiole, and stem explants. To the best of our knowledge, the present study is the first report on the development of a simple and rapid in vitro regeneration system for direct and indirect regeneration of S. araxensis.
Materials and methods
Media and culture conditions
Half-strength Murashige and Skoog (MS) medium enriched with 3% sucrose supplemented with different combinations and concentrations of BAP (0.5, 1, 1.5, and 2 mg/l) plus TDZ (0.1, 0.5, 1, and 1.5 mg/l), IBA (0.1, 0.5, 1, and 1.5 mg/l), and NAA (0, 0.1, 0.5, and mg/l) was used as culture media. Half-strength MS medium supplemented with 0.5 mg/l gibberellic acid (GA3) was used for shoot elongation and multiple shoot production. Root induction was performed in half-strength MS medium enriched with 0.5 mg/l IBA (Gharari et al., 2021b). The pH of the medium was adjusted to 5.7–5.8 before the addition of agar (0.8% w/v), and the medium was then sterilized for 15 min at 121°C.
Surface sterilization, callus induction, and shoot regeneration
This study was conducted at the Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran. S. araxensis seeds were collected from their natural habitats in the surroundings of Jolfa city, subdivision in the East Azerbaijan province of Iran in July 2018 and were then identified by Dr. Alireza Yazdinezhad from the Department of Pharmacognosy, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran (voucher specimen number: 1316). Healthy and uniform seeds were washed under running tap water for 15 min, surface sterilized with 70% ethanol for 15 s, and then immersed for 10 min in 10% bleach (NaOCl) with 2 drops of Tween-20, followed by rinsing three times for 3 min each in sterile water. For seed germination, the sterilized seeds were transferred into sterile plates and then cold stratified at 4°C for 4 weeks. The seeds were cultivated on agarsolidified water for germination at 24 ± 2°C for 16 h photoperiod (light intensity of 80.5 μmol m-2 ∙ s-1) and 85% relative humidity. After germination, the seedlings were transferred into half-strength MS medium. Petiole, leaf, and stem segments were used as explants for callus formation and shoot organogenesis. They were cut out from 2-month-old seedlings grown under in vitro conditions. Explants (1 × 1 cm) were inoculated horizontally on half-strength MS medium containing various concentrations and combinations of plant growth regulators (PGRs). Explants grown on half-strength MS medium without the addition of PGRs were used as control cultures.
Shoot elongation and proliferation
After direct shoot induction from stem explants and indirect shoot organogenesis from stem-derived callus, micro-shoots (buds) were excised and subcultured on half-strength MS medium supplemented with 0.5 mg/l GA3 at regular intervals of 2 weeks for 2 months to achieve shoot elongation and avoid vitrification. The frequency of in vitro shoot organogenesis and the number of shoots per explant were then recorded.
In vitro rooting of shoots and acclimatization of plantlets
The obtained induced shoots were excised and transferred into half-strength solid MS medium supplemented with 0.5 mg/l IBA in a growth chamber maintained at 25± 2°C, 85% relative humidity, and 16 h photoperiod (Gharari et al., 2021b). After 4 weeks, the rooted plantlets were removed from the medium and gently washed with sterile distilled water to remove traces of the adhered medium. The plantlets were then transferred to plastic cups containing peat moss covered with transparent polythene bags to create a high relative humidity, maintained in the growth chamber at 25°C for 18 days, and watered every alternate day with 1/4 liquid MS basal medium. After 7 days, the transparent polyethylene bags were slowly cut and slightly removed to achieve hardening. Following acclimatization, the plants were transferred to bigger pots containing sand and normal garden soil in the ratio of 1 : 2 and kept under greenhouse conditions for more growth.
Statistical analysis
Experiments were conducted under a completely randomized design. Each treatment contained 10 explants per culture pellet and three culture pellets per treatment. Variance analysis was performed with IBM SPSS Statistics 22.0 software. Mean values were compared using Duncan’s multiple range tests. Graphs were created using Microsoft Excel 2013.
Results
Effect of BAP and TDZ on callus induction and shoot organogenesis
The highest frequency of callus induction (100%) was observed in stem explants in all the tested PGR concentrations (Table 1). However, for petiole and leaf explants, 100% callus induction was obtained only on media containing the lowest BAP concentration (0.5 mg/l) (Table 1).
Table 1
Effect of BAP in combination with TDZ, NAA, and IBA on callus induction in S. araxensis explants
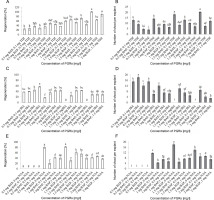
No direct shoot organogenesis was observed in the combined treatments of BAP and TDZ. Figures 1A–D represent the formation of compact and light green calli in callus cultures derived from stem explants, followed by the development of micro-shoot primordial on their surfaces (Fig. 1A–D). The micro-shoots started to develop shoots in the subsequent subcultures in cultures containing 0.5 mg/l GA3. Although micro-shoots were induced from all stem-derived calli containing BAP and TDZ, it is evident that a higher concentration of BAP (2 mg/l) combined with 0.5 mg/l TDZ significantly increased the percentage of shoot induction (100%) and shoot numbers per explant (20.33) (Fig. 2A, Fig. 2B). Root formation in induced shoots was observed after transferring the shoots to half-strength MS medium supplemented with 0.5 mg/l IBA.
Fig. 1
Shoot organogenesis of S. araxensis using different concentrations and combinations of BAP (0.5, 1, 1.5, and 2 mg/l) with TDZ (0.1, 0.5, 1, and 1.5 mg/l), IBA (0.1, 0.5, 1, and 1.5 mg/l), and NAA (0, 0.1, 0.5, and 1 mg/l). A–D) indirect shoot organogenesis from stem-derived callus using BAP plus TDZ; E–H) direct (E) and indirect shoot organogenesis (F–H) from stem explants using BAP plus IBA; I–L) callus induction in petiole explants (I) and indirect shoot organogenesis from stem-derived callus using BAP plus NAA (J–L)
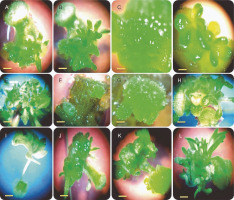
Fig. 2
Shoot organogenesis of S. araxensis using different concentrations and combinations of BAP with TDZ, IBA, and NAA. A, B) frequency of regeneration and shoot number per explant using BAP plus TDZ; C, D) frequency of shoot organogenesis and shoot number per explant using BAP plus IBA; E, F) frequency of regeneration and shoot number per explant using BAP plus NAA
Effect of BAP and IBA on callus induction and shoot organogenesis
After transferring of stem and petiole tissues to halfstrength MS medium supplemented with various combinations of BAP and IBA, callus formation occurred within 10 days (Fig. 1E–H), while no callus induction was found in leaf explants (Table 1). In most treatments, the callus induction frequency was higher in stem explants than in petiole explants (Table 1).
Although callus induction occurred in both stem and petiole explants, shoot organogenesis was observed only in stem explants (Fig. 2C). The maximum indirect shoot organogenesis frequency (57%) from callus was achieved when half-strength MS medium supplemented with higher concentration of IBA (1.5 mg/l) and lower concentration of BAP (0.5 mg/l) was inoculated with compact callus obtained from stems (Fig. 2C). Figures 1F–H represent the callus formation and shoot organogenesis from stem explants cultured on half-strength MS medium fortified with BAP and IBA (Fig. 1F–H). The maximum number of indirect regenerated shoots (15) was obtained from calli cultured in media supplemented with 0.5 mg/l BAP and 0.1 and 1.5 mg/l IBA (Fig. 2E). Direct shoot organogenesis was observed following the culture of S. araxensis stem-derived calli on half-strength MS medium containing 0.5 mg/l BAP and 0.5 mg/l IBA (40%) (Fig. 1E, 2C). It was observed that the callus culture fortified with 0.5 mg/l BAP and 0.5 mg/l IBA, which caused direct regeneration from stem-derived calli, yielded the maximum number of shoots (18) (Fig. 2C). In the absence of PGRs, neither callus production nor plant regeneration occurred in explants.
Effect of BAP and NAA on callus induction and shoot organogenesis
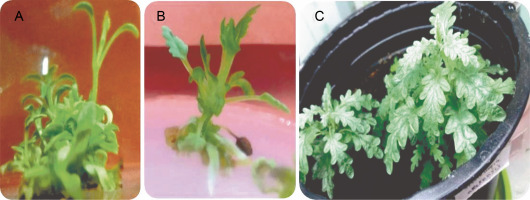
The results of callus induction using BAP and NAA showed that callus formation occurred in the stem and petiole explants in all cultures 1 week after incubation. In terms of callus induction, the frequency of callus production from stem explants was higher than that from petiole explants (Table 1). The induced light green callus had higher shoot induction and proliferation frequency. Neither callus induction nor organogenesis was observed in leaf explants. Shoot organogenesis from stem-derived calli was observed in all cultures, except when grown on a medium containing 0.5 mg/l BAP in combination with NAA at all concentrations (0, 0.1, 0.5, and 1 mg/l). Petiole-derived callus did not show any organogenesis in cultures containing PGRs (Fig. 1I). Figures 2J–L show the various stages of indirect shoot organogenesis, including callus induction, shoot organogenesis, and shoot elongation, from stem-derived callus cultured in medium supplemented with BAP plus NAA (Fig. 1J–L). BAP alone at 1.5 and 1 mg/l concentrations induced shoot formation at the rate of 81.6 and 80%, respectively, which correspondingly stimulated the growth of 18 and 11.6 shoots per stem explant on average (Fig. 2E, Fig. 2F). The well-developed foliated micro-shoots (3–4 cm) were elongated on a medium fortified with 0.5 mg/l GA3 (Fig. 3A) and then successfully rooted in half-strength MS medium augmented with 0.5 mg/l IBA (Fig. 3B). The rooted plants were then acclimatized with a survival rate of 100% (Fig. 3C).
Discussion
Because of improper harvesting, overexploitation, habitat destruction, and poor seed sets, there are significant challenges in the propagation and regeneration of medicinal plants (Shafi et al., 2021). Scutellaria is a widely distributed, subcosmopolitan genus comprising around 360 species with several therapeutic properties such as antitumor, hepatoprotective, antioxidant, anti-inflammatory, anticonvulsant, antibacterial, and antiviral activities (Shang et al., 2010). Although there are a few reports on in vitro organogenesis and micro-propagation of Scutellaria species (Trivedi et al., 2011; Zakaria et al., 2020; Gharari et al., 2021b; Irvin et al., 2021), there is no report on the in vitro regeneration and propagation of S. araxensis.
Because S. araxensis is a medicinally important plant, the development of a robust organogenesis and in vitro propagation protocol for this plant is necessary. Accordingly, the present study was conducted to establish an efficient in vitro shoot organogenesis and plant regeneration protocol through multiple shoot induction from three types of explants, namely stem, petiole, and leaf of S. araxensis, by using different concentrations and combinations of BAP with TDZ, NAA, and IBA. The results showed that the addition of BAP combined with TDZ in half-strength MS medium strongly induced the generation of adventitious shoots than the use of BAP alone (Fig. 2A, Fig. 2B). However, our results are in contrast with those reported by Rahayu and Adil (2012) on shoot organogenesis of Curcuma xanthorrhiza from shoot explants in a medium containing BAP and TDZ. Conversely, a recent study conducted by Gharari et al. (2021b) revealed that the use of BAP combined with TDZ in half-strength MS medium has a stronger effect on the percentage (100%) of Scutellaria bornmuelleri shoot organogenesis than TDZ when applied alone (93.3%) (Gharari et al., 2021b); thus, these findings support the result of the present study. A synergistic effect between TDZ and BAP was observed in S. bornmuelleri organogenesis (Gharari et al., 2021b).
It is well known that cytokinins initiate the de novo shoot apical meristem (SAM) formation in tissue cultures. Based on the analogy of the endocrine system of animals, a model has been proposed for the functioning of cytokinins in plant cells. The CREB-binding protein (CBP) receptor has two different binding sites for BAP and TDZ. Both BAP and TDZ can bind to a CBP receptor; the naturally occurring adenine-type cytokinin (BAP) binds to one of the binding sites, while the phenyl ureatype cytokinin (TDZ) binds to the other binding site (Gharari et al., 2021b). According to Gharari et al. (2021b), the combined use of BAP and TDZ can improve the formation of adventitious shoots, probably due to the active occupation of both CBP sites by BAP and TDZ (Gharari et al., 2021b). As reported by Guo et al. (2011), TDZ may directly or indirectly modify endogenous PGRs such as BAP and produce responses necessary for the division/regeneration of cell/tissue. It has been proposed that in tissue culture systems, the synergetic effect of TDZ and the adenine-type cytokinin is either due to binding of the adenine-type cytokinin (BAP) to CBP and the induction of the well-known cytokinin effect on shoot formation by TDZ or probably due to TDZ-induced synthesis and accumulation of endogenous BAP (Erland et al. 2020). This clarifies that the appropriate combination and concentration of PGRs are required for callus formation and plant organogenesis from explants in culture media.
The combinations of BAP plus IBA PGRs had significant effects on shoot organogenesis frequency and shoot multiplication (P < 0.01). The frequency of shoot organogenesis from stem explants varied between 17 and 57%. High frequency of shoot organogenesis was achieved from half-strength MS medium containing 2 mg/l BAP in combination with 0.1 mg/l IBA (Fig. 2C). The maximum shoot number per explant (18 shoots) was observed in 1 mg/l BAP and 0.1 mg/l IBA treatment through direct shoot regeneration from stem explants (Fig. 2D). Successful promotion of shoot regeneration and multiplication using combinations of BAP and IBA have been reported in various medicinal plants such as Sebri pear (Karimpour et al., 2013), Fragaria x ananassa Duch. (Madumali et al., 2019), Juniperus procera Hoechst. Ex Endl. (Salih et al., 2021), Ficus religiosa (Hesami et al., 2018), and Securidaca longipedunculata (Lijalem and Feyissa, 2020).
Bisht et al. (2012) investigated the in vitro propagation of Polygonatum verticillatum and suggested that the favorable impact of cytokinin and auxin combination is due to increased RNA synthesis. The applied combination induces a peak of RNA synthesis. This peak is associated with the appearance of first bud primordial, thus conditioning bud formation (Bisht et al., 2012).
BAP is known to efficiently induce shoot regeneration and shoot multiplication in different species of plants, such as Colocasia esculenta L. Schott (Kepue et al., 2021), Argania spinosa (L.) (Amghar et al., 2021), Citrus x meyeri (Haradzi et al., 2021), and Citrus jambhiri (Sharma and Roy, 2020). The results of the present study revealed that the half-strength MS medium containing 1.5 and 1 mg/l of BAP alone induced the highest frequency of shoot multiplication (81.6 and 80%, respectively) (Fig. 2E, Fig. 2F). In addition, BAP with NAA was found to have the best synergistic effect on shoot regeneration in Chenopodium quinoa (Hesami et al., 2016), Cucumis trigonus (Mali and Chavan, 2016), and Beta vulgaris (Yildiz et al., 2013). The synergistic effect for shoot organogenesis (60%) and multiplication (9.33 shoots per explant) from stem explants was found to be more effective for the combination of 1.5 mg/l BAP and 0.5 mg/l NAA, and 2 mg/l BAP combined with 0.5 mg/l NAA, respectively (Fig. 2E, Fig. 2F).
Conclusions
In conclusion, the present study showed that the synergistic effect of two cytokinins, namely BAP and TDZ, successfully induced in vitro shoot organogenesis of S. araxensis. The synergistic effect of BAP (0.5-2 mg/l) combined with TDZ (0.1–1.5 mg/l) was more effective than that of BAP combined with auxins (NAA and IBA) in terms of shoot organogenesis and number of shoots per explant. Among the two auxins tested in this study, NAA could more successfully induce shoot organogenesis than IBA, while shoot formation was promoted significantly by both the PGRs. Regarding the use of different explants and treatments in this study, stems were the best explants for both direct and indirect shoot organogenesis. Moreover, while the combination of 1 mg/l BAP and 0.1 mg/l IBA was the best treatment for direct organogenesis, the combination of 2 mg/l BAP with 0.5 and 1.5 mg/l TDZ had the best effect on indirect shoot organogenesis. The individual and combined effects of cytokinins and auxins on in vitro S. araxensis shoot organogenesis could be used for the regeneration of other important Scutellaria species.