Introduction
In transgenic plant development, induction of competency and transformation of Agrobacterium tumefaciens is a crucial step. In the literature, various techniques have been reported for the transformation of Agrobacterium, including triparental mating, electroporation, and freeze-thaw method (Wise et al., 2006; Kaman-Toth et al., 2018). Triparental mating is a time-consuming process as it involves two Escherichia coli strains in Agrobacterium transformation. Though electroporation has a high transformation efficiency, the cost of equipment and the requirement of high cell density are its major limiting factors (Rebersek and Miklavcic, 2011). The most convenient and less expensive method is the freeze-thaw technique, but poor transformation efficiency is its major limitation (Karthik et al., 2020). Therefore, there is still a need for methods to improve the efficiency of the introduction of DNA into A. tumefaciens.
To induce competency, the conventional transformation process involves chemical modification of the cell membrane, followed by physical uptake of foreign DNA (Asif et al., 2017). Using chemicals such as calcium chloride and by decreasing the temperature, transient pores are induced on the cell surface due to the loss of protein and lipid, followed by a heat shock process. Depolarization of the cell membrane reduces the repulsion between the negatively charged DNA molecule and the cell membrane and facilitates the uptake of the DNA molecule (Panja et al., 2008). Nanoparticle-mediated gene transfer methods have the potential to directly transfer DNA into the cells and thus can achieve stable integration and rapid expression of the transgene (Rai et al., 2015). To address delivery challenges, gene transfer using nanoparticles has evolved as a key transformational tool, and so its application in plant genetic engineering has also increased (Cunningham et al., 2018). In a previous study, silver nanoparticles (AgNPs) were used as an efficient gene carrier in a biolistic gene delivery process in Nicotiana tabacum (Rajkumari et al., 2021). Nanoparticle-based carriers such as gold, silver, and hydroxyapatite were reported to increase the transformation efficiency of bacteria (Chatterjee and Sarkar, 2014; Deshmukh et al., 2019; Nagamani et al., 2019). AgNPs can disrupt the cell membrane and penetrate the cell by increasing the porosity of the cell membrane (Dong et al., 2019). It has been reported that the addition of AgNPs increases the transformation efficiency in Escherichia coli and aids in the uptake of exogenous DNA by bacterial cells (Nagamani et al., 2019). AgNPs at concentrations below toxic levels for Agrobacterium can be used to initiate interactions with the biological membrane, which would aid in the uptake of foreign DNA. In this study, a technique for improving the transformation of Agrobacterium using AgNPs was proposed, and the efficiency of AgNPs in improving the transformation of Agrobacterium is reported for the first time.
Materials and methods
Chemicals
All the chemicals used for the preparation of the culture medium were of analytical grade and were obtained from Sisco Research Laboratories, India. Antibiotics were purchased from HiMedia Laboratories, Mumbai, India. AgNPs of 100 nm particle size were obtained from Sigma Aldrich, USA.
Toxicity assay
AgNPs (Sigma Aldrich, USA) of different concentrations (0.01, 1, 5, 10, and 20 mg/l) were prepared by dissolving the commercial product in sterile deionized double-distilled water. The toxicity of AgNPs on Agrobacterium was determined in accordance with the protocol of Ivask et al. (2014). An overnight culture of A. tumefaciens (strain EHA105) was diluted in a 1: 50 ratio in Luria-Bertani (LB) broth and cultured until the exponential growth phase was reached (OD600 = 1). The bacterial culture (1 ml) was pelleted at 5000 rpm for 5 min and resuspended in 1 ml of sterile deionized double-distilled water. Then, 200 μl of bacterial suspension was mixed separately with 200 μl of each of the different concentrations of AgNPs in sterile tubes and incubated at 28°C for 4 h. After incubation, serial dilutions of the cultures were made, and 100 μl was spread on LB agar plates containing rifampicin (100 μg/ml). The half-maximal effective concentration (EC50) value of AgNPs was estimated via Probit analysis using the statistical package SPSS (https://www.ibm.com/in-en/analytics/spss-statistics-software).
Induction of competency and transformation of bacterial cells
As presented in Table 1, treatments for the induction of competency in bacterial cells were designed by modifying the conventional technique involving both calcium chloride and freeze-thaw techniques. Treatments T1–T4 included different concentrations of AgNPs alone, T5–T8 included AgNPs with calcium chloride, T9–T12 included AgNPs with freeze-thaw, and T13–T16 included the addition of AgNPs with the conventional calcium chloride freeze-thaw technique. The conventional calcium chloride freeze-thaw technique (T17) served as the positive control (Table 1). T18 was the negative control in which bacterial cells with plasmid were used without the calcium chloride freeze-thaw treatment. Treatments were conducted in duplicate.
Table 1
Different treatments for induction of competency and transformation of Agrobacterium tumefaciens EHA105
Four concentrations of AgNPs below the established EC50 value, i.e., 0.01, 0.5, 1, and 2 mg/l, were tested. For the transformation experiments, A. tumefaciens (strain EHA105) cells in the early exponential phase (OD600 0.3–0.4) were made competent. The cells were harvested by centrifugation at 4000 rpm for 10 min at 4°C in a microcentrifuge apparatus. In the treatments involving AgNPs alone, the harvested bacterial cells were resuspended in 200 μl each of the different concentrations of AgNPs and incubated at 28°C for 60 min with shaking. In treatments involving AgNPs and calcium chloride, 100 μl of 20 mM calcium chloride was added along with 100 μl of different concentrations of AgNPs to the cell suspension and incubated on ice for 30 min.
Freshly prepared competent cells were transformed using pART27 (11.6 kb) obtained from Commonwealth Scientific and Industrial Research Organisation, Australia.
To 200 μl of competent cells, 1 μl of pART27 containing 1 μg of plasmid DNA was added and incubated on ice for 15 min; then, 1 ml of LB broth was added and again incubated at 28°C for 2 h at 180 rpm. In treatments involving freeze-thawing, after the addition of plasmid and incubation on ice for 15 min, the suspension was frozen in liquid nitrogen for 5 min, followed by thawing at 37°C for 5 min. For the conventional calcium chloride freezethaw technique (T17 maintained as the positive control), the protocol of Weigel and Glazebrook (2006) was followed.
The success of transformation was confirmed by plating the transformed cells on LB agar containing rifampicin (100 μg/ml) and spectinomycin (100 μg/ml) as selectable markers. All the treatments were conducted in duplicate. Transformation efficiency was assessed by counting the number of transformants, i.e., CFU obtained per μg of plasmid DNA. Transformation efficiencies of different treatments were compared to achieve statistical significance. Using analysis of variance (ANOVA), critical difference (CD) values at 5% level of significance were calculated.
Confirmation of transformation by colony PCR
Using colony polymerase chain reaction (PCR) analysis with a gene-specific primer for neomycin phosphotransferase (npt II) gene, transformants were tested for the presence of the binary vector, pART27, in A. tumefaciens. A 475-bp fragment of npt II gene was amplified using primers 5′−GGTGCCCTGAATGAACTG−3′ and 5′−TAGCCAACGCTATGTCCT−3′. PCR was carried out in a 20-μl reaction mixture containing 200 μM dNTPs, 10 pM of each primer, 1 U of Taq polymerase, and 1 × Taq polymerase buffer. A single transformed bacterial colony was added to the reaction mix. Cycling conditions were as follows: a denaturing step at 95°C for 5 min; 30 cycles at 95°C for 30 s, annealing for 30 s at 55°C, and elongation for 30 s at 72°C; and a final extension at 72°C for 5 min. PCR products were separated on 1.2% (w/v) agarose gel and stained with ethidium bromide (0.5 μg/ml).
Results
Toxicity assay
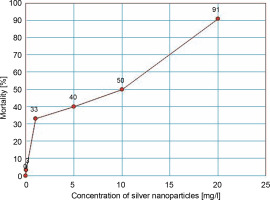
As shown in Table 2, A. tumefaciens EHA105 showed a dose-dependent growth response to different concentrations of AgNPs. The maximum inhibition (91%) of growth was observed with 20 mg/l, and the minimum inhibition (3.3%) with 0.01 mg/l. Based on the findings of the Probit analysis of inhibition percentage versus concentration of AgNPs, the EC50 of AgNPs was 8.707 mg/l (Fig. 1).
Induction of competency and transformation of bacterial cells
According to the above findings, a significant difference in the transformation efficiency in A. tumefaciens EHA105 harboring pART27 was observed when subjected to different treatments (Table 3). The CD obtained using ANOVA was 0.021. The treatments involving AgNPs alone at six different concentrations (T1–T4) showed significantly higher transformation efficiencies, ranging from 2.78 to 2.91 log CFU/μg of DNA, compared with the conventional technique (2.31 log CFU/μg of DNA).
Table 3
Transformation efficiency of A. tumefaciens EHA105 cells made competent with different treatments
| Treatment * | Transformation efficiency [log CFU/μg of DNA] ** |
|---|---|
| T1 | 2.86 d |
| T2 | 2.86 d |
| T3 | 2.91 c |
| T 4 | 2.78 f |
| T5 | 3.33 a |
| T6 | 3.34 a |
| T7 | 3.27 b |
| T8 | 3.25 b |
| T9 | 2.32 h |
| T10 | 2.30 h |
| T11 | 2.18 i |
| T12 | 2.10 j |
| T13 | 2.81 e |
| T14 | 2.84 d |
| T15 | 2.79 ef |
| T16 | 2.78 f |
| T17 | 2.31 h |
| T18 (negative control) | 0 |
| CD (0.05) = 0.027 | |
* treatment as in Table 1;
Among the different treatments, the combination of AgNPs and calcium chloride without the freeze-thaw stage (T5–T8) showed a significantly higher transformation efficiency compared with the conventional technique. The maximum transformation efficiency was achieved by using 0.5 mg/l AgNPs with 20 mM calcium chloride (3.34 log CFU/μg of DNA), which was on par with that obtained with 0.01 mg/l AgNPs with 20 mM calcium chloride (3.33 log CFU/μg of DNA).
When calcium chloride in the conventional calcium chloride freeze-thaw technique was replaced with AgNPs (T9–T12), no significant improvement in transformation efficiency was observed (2.32, 2.3, 2.18, and 2.1 log CFU/μg of DNA) compared with the conventional technique (2.31 log CFU/μg of DNA). When AgNPs were added to the conventional calcium chloride freeze-thaw technique (T13–T16), a significantly higher transformation efficiency was achieved (2.81, 2.84, 2.79, and 2.78 log CFU/μg of DNA) compared with the conventional calcium chloride freeze-thaw technique; however, it was lower compared with that of the treatment in which the combination of AgNPs and calcium chloride was used.
As observed from the results of colony PCR of the transformed colonies from different treatments, DNA bands of the predicted size (475 bp) in agarose gel electrophoresis were obtained, which confirms the success of transformation in all the treatments (Fig. 2).
Discussion
As mentioned earlier, in transgenic plant development, induction of competency and transformation of Agrobacterium is a crucial step. Due to the inherent limitations of the currently available methods, such as triparental mating, electroporation, and freeze-thaw technique, attempts to improve the efficiency of introducing DNA into Agrobacterium are in demand.
Since AgNPs can induce pores on the bacterial cell membrane (Mikhailova, 2020), their efficacy in improving the conventional calcium chloride freeze-thaw technique for the transformation of A. tumefaciens was explored in this study. In different treatments tested, concentrations of AgNPs below the EC50 value were used. As shown in Fig. 1, a dose-dependent toxicity response of AgNPs on A. tumefaciens was noticed. A similar response of AgNPs has been reported in E. coli and Staphylococcus capitis in previous studies (Kim et al., 2007; Shrivastava et al., 2007; Amin et al., 2009).
All treatments involving AgNPs exhibited significant variations in transformation efficiency. Treatments with AgNPs (0.01, 0.5, 1, and 2 mg/l) along with calcium chloride (20 mM) showed higher transformation efficiencies (3.25–3.34 log CFU/μg of DNA) compared with all other treatments. The transformation efficiency of treatments with AgNPs along with calcium chloride was tenfold higher than that of the conventional technique (Table 3). This is because the combined action of AgNPs and calcium chloride might have increased the permeability of the membrane, thus leading to the enhanced uptake of plasmid DNA (Nagamani et al., 2019). Wang et al. (2011) reported that competent cells of A. tumefaciens strains EHA105 and LBA4404 prepared using calcium chloride in combination with dimethyl sulfoxide or polyethylene glycol 4 000 showed significantly higher transformation efficiency. The toxicity of AgNPs might be reduced by the action of chloride ions from calcium chloride, thus resulting in higher transformation efficiency. Chambers et al. (2014) reported that in the presence of a high concentration of chloride ions, E. coli showed increased tolerance to AgNPs due to the dissolution of silver ions and the formation of less toxic silver chloride.
The transformation efficiency of treatments with AgNPs alone was threefold higher (2.78–2.91 log CFU/μg of DNA) than that of the calcium chloride freeze-thaw technique (2.31 log CFU/μg of DNA). Nagamani et al. (2019) reported that E. coli cells treated with 100 nm AgNPs at a concentration of 1 mg/l exhibited higher transformation efficiency (7.9 × 104 CFU/ng of DNA) compared with the calcium chloride method (2.3 × 103 CFU/ng of DNA). The increase in the transformation efficiency might be due to the enhanced activity of AgNPs on bacterial membrane, resulting in a larger pore size together with a larger surface area of AgNPs facilitating a greater plasmid uptake. According to Weston et al. (1981), cations reduce the repulsive force between the negatively charged DNA and the outer membrane and facilitate better DNA membrane contact. Ashmore et al. (2018) reported that E. coli cells treated with AgNPs exhibited disruption at the surface of the cells characterized by pits in the cell walls.
A transformation efficiency of 2.91 log CFU/μg of DNA was achieved for the concentration of 1 mg/l, whereas with increase in concentration of AgNPs to 6 mg/l, a comparatively low transformation efficiency was recorded (2.74 log CFU/μg of DNA). This may be attributable to hindrance to DNA replication as well as binding of AgNPs to the thiol groups of proteins, leading to their inactivation (Tang and Zheng, 2018). Feng et al. (2000) reported that silver ions condense the DNA molecule, thus resulting in reduced replication in E. coli. Yan et al. (2018) confirmed by bioinformatics analysis that the antibacterial mechanism of AgNPs involves the disruption of the cell membrane as well as the generation of intracellular reactive oxygen species due to the release of silver ions.
Transformation efficiency of the AgNPs along with freeze-thaw technique was significantly lower or comparable to the conventional technique (Table 3). This lower transformation efficiency might be due to the toxicity of AgNPs together with the stress imposed by freeze-thawing.
The method involving the use of AgNPs along with calcium chloride and freeze-thawing resulted in a threefold increase in transformation efficiency compared with the conventional calcium chloride freeze-thaw technique. This might be attributable to the combined action of AgNPs and calcium chloride. The action of chloride ions might have nullified the toxicity effect of AgNPs. The oxidative dissolution of AgNPs, by the action of oxychloride ions, may form less reactive silver chloride (Garg et al., 2016). However, transformation efficacy significantly improved when freeze-thawing was avoided (Table 3).
Conclusions
This is the first report on the use of AgNPs in the transformation of A. tumefaciens. The efficiency of the conventional calcium chloride freeze-thaw technique for Agrobacterium transformation is low as it yields only very few transformed colonies. The use of AgNPs at a concentration of 0.01 mg/l along with 20 mM calcium chloride is found to be an economically viable method as it increases the transformation efficiency in A. tumefaciens by tenfold. This increased transformation efficiency might be due to the action of AgNPs on the cell membrane coupled with the effect of calcium chloride in neutralizing the deleterious effect of the nanoparticles.