Introduction
Maize is an important crop in Africa and the rest of the world. It is one of the three main nutritious crops that serves as a staple diet for more than half of the world’s population (FAO, 2009; Macauley, 2015). In Benin, maize is grown as a strategic crop in terms of food security and economic profitability, and consequently, it is regarded by the government of Benin as the main crop whose production needs to be intensified in order to achieve food self-sufficiency (Toléba-Seïdou et al., 2015; APRM, 2017). At the national level, maize production has increased from 864 698 t in 2005 to 1 600 000 t in 2018 (Gogan et al., 2018), albeit with low yields per hectare (< 3 t/ha), which is below the potential yield of the plant (3 to 6 t/ha) despite the favorable ecology for its production in all agro-ecological zones (Azontondé et al., 2010; Adjanohoun et al., 2012; Sikirou et al., 2019). One of the main factors that affects soil fertility in modern agriculture is abiotic stress caused by climate change, soil salinity, and soil degradation, which has a severe negative effect on food availability (Maheshwari, 2012; Goyal and Manoharachary, 2014). In Benin, the phenomenon of soil degradation is increasing, particularly in the central and northern regions dominated by ferruginous soils (Amadji and Migan 2001). This situation contributes to the depletion of soil organic matter and nutrients essential for plant development. The resulting decline in soil fertility is a major concern for both farmers and allied services, which has ultimately led to a full-fledged project in the National Agricultural Research Programme (NARP) adopted by the Government in the Council of Ministers on June 13, 2018 (INRAB, 2018). Current agricultural systems implemented to ensure better productivity depend heavily on the continuous application of mineral fertilizers, mainly nitrogen (N), phosphorus (P), and potassium (K), which contribute to the deterioration of soil biological fertility due to the loss of microbial population beneficial for plants (Gyaneshwar et al., 2002; Mahanty et al., 2017). The presence and high activity of microorganisms is often considered as an indicator of good soil fertility (Patra et al., 2008; De Souza et al., 2015). To meet the abovementioned challenges, the development of different production techniques based on the importance of different nutrient sources and the potential of soil microorganisms, especially plant growth-promoting rhizobacteria (PGPR), has become an urgent necessity. Indeed, a microbial inoculation technology using PGPRs has been proposed to improve crop production yields in several regions of the world (Rashid et al., 2016; Dicko et al., 2018; Zahir et al., 2018). PGPRs influence plant health and productivity through various mechanisms. The direct mechanism involves soil improvement and the production of substances needed for plant growth, which enhances fertility by mobilizing soil minerals. These improvements include the supply of growth regulators and essential minerals such as N and P. The indirect mechanism involves production of biocontrol agents that inactivate or kill pathogens, thereby providing a healthy growth environment for plants. Moreover, bacteria can also indirectly improve plant growth by suppressing pathogens and increasing plant innate immunity against pathogens (Tabassum et al., 2017; Saikia et al., 2018; Naik et al., 2019). Currently, PGPRs are the most widely used tools because of their effectiveness and relatively low cost in managing and improving soil productivity in sustainable harmony with populations of beneficial and pathogenic soil microorganisms and human health (Batool et al., 2014; Gontia-Mishra et al., 2016; Ahmad et al., 2019 ). The present study assessed the effect of inoculation of PGPR isolated from the maize rhizosphere in central and northern Benin and the intake of different doses of NPK-Urea on maize productivity on poor ferruginous soil in central Benin with a view to their valorization in the production system for ecological and sustainable agriculture.
Materials and methods
Characteristics of rhizobacteria strains
The rhizobactéries strains Bacillus panthothenicus, Bacillus thuringiensis, Pseudomonas putida, Pseudomonas syringae, and Serratia marcescens used in this study were selected on the basis of their ability to promote greenhouse maize growth parameters (Amogou et al., 2019). The strains were isolated and characterized from the maize rhizosphere of different agro-ecological zones of central and northern Benin by Agbodjato et al. (2015) and stored at –86°C in Muller-Hinton broth with glycerol (10%) at the Laboratory of Biology and Molecular Typing in Microbiology, the University of Abomey Calavi, Republic of Benin.
Preparation of the inoculum
After culturing the strains of Pseudomonas on Middle King B (Guiraud and Galzy, 1994) and those of Bacillus and Serratia on Nutritive Agar (Wahyudi et al., 2011; Aparna and Sarada, 2012), the corresponding inocula of the different PGPRs were obtained by growing in nutrient culture (Mueller Hinton Broth) for 24 h at 30°C. Bacterial culture concentrations were adjusted to approximately 1 × 108 CFU/ml (DO 0.45 to 610 nm) with a spectrophotometer using the method of Govindappa et al. (2011).
Description of the study site
The trial was conducted in Miniffi in central Benin in the municipality of Dassa-Zoumè (Fig. 1). It is home to depleted tropical ferruginous soil with low water supply. The location is characterized by a Sudano-Guinean tropical climate with an annual rainfall and large temperature variations with an annual average of around 28°C; the average annual rainfall is 1 481 mm, and the average annual potential evapotranspiration is 1 648 mm (Balogoun, 2012).
Experimental design
The experiment was conducted in a split-split plot-based randomized complete block design with three replications. The large plot factor was the fertilizer dose and the small plot factor was the concentration of PGPR. A total of 22 treatments were compared, with three repetitions each. The plots were divided into three large blocks that represented the repetitions. Each block was divided into four large parcels, three of which had 7 small plots and the fourth had only one small plot; seeds were randomly sown on each block under the following conditions: without inoculation, with inoculation, and with increasing doses of NPK mineral fertilizer input. Table 1 provides details of the factors and treatments.
Table 1
Evaluation of doses of NPK fertilizer (0, 25, 50, and 100% with no PGPRs) and PGPRs (without PGPR, B. thu., B. pan., P. put., P. syr., and S. mar. )
Sowing, inoculation of corn seeds, and maintenance of plots
The maize variety EVDT 97 STR C1 provided by the National Institute of Agricultural Research in Benin was used. This is an early 90-day variety, with a potential yield of grain ranging from 4 to 5 t/ha in a pleasant environment. The seeds of this corn are white and toothed, and the texture is mid-farinaceous and mid-vitreous. The EVDT 97 STR C1 variety has a good resistance to American rust, striation, Helminthosporium species, Curvularia species, and drought (Yallou et al., 2010). Two seeds of each treatment were sown at the depth of 5 cm. The seeds were inoculated with 10 ml of bacterial suspension according to each treatment, and the opening was immediately closed. Two weeding towers were carried out 12 days after sowing (DAS) and 42 DAS for the maintenance of the different plots.
Data collection
The height and diameter at the collar of 12 plants were measured every 15 days by using a tape meter and a sliding foot scale after thinning of the plants up to 60 DAS.
In addition, the leaf surface of the plants was assessed 60 DAS according to the method described by Ruget et al. (1996). The number of feet of corn was counted by an elemental plot following perfect drying of the spaths of the ears, and 12 plants located on the three central lines were removed to evaluate yield parameters. For this purpose, the ears of the plants were harvested, and the fresh aerial parts and underground biomass were separated and weighed. The dry weight of the biomass was measured in the laboratory, after drying in a stove at 65°C for at least 72 h. After ginning, the cob stalks of the 12 previously plucked corn plants were weighed with a scale (highland HCB 302, max: 3001 g) with an accuracy of 0.1 g. The relative humidity of the seeds was determined using a moisture meter (LDS-1F). On each elementary plot, the average grain yield was calculated using the formula of Valdés et al. (2013):
where R is the average yield of maize, expressed in t/ha; P is the fresh weight of maize per elementary computing area, expressed in kg SI is the surface interpretable expressed in m2; H is the moisture of the grains, expressed in percentage (%); 100 000 represents the conversion of ha in m2, and 1000 represents the conversion of ton (t) in kg.
Chemical analysis of the soil at the experimental site
The composite soil samples obtained prior to the implementation of the trial were analyzed at the Laboratory of Soil, Water and Environment Sciences (LSSEE) of the National Institute of Agricultural Research in Benin (INRAB). These analyses included pH (Kumar et al., 2012), organic carbon (Lo et al., 2011), assimilable phosphorus (Olsen et Sommer, 1982), nitrogen (Bremner, 1996), potassium (Knudsen et al., 1982), and cation (Thomas, 1982).
Statistical analysis
For growth parameters such as height and collar diameter, a mixed linear model on longitudinal data was considered with the blocks constituting the random factor and the treatments, the fixed factor. The empty model with no factor was tested before choosing the unconditional growth model and the full conditional growth model. By using this model, a simple analysis of variance (ANOVA) allowed to conclude on the significance of the PGPR and NPK dose factors, the time factor (day after sowing), and their interactions. Growth curves were constructed from the adjusted averages of the treatment factor levels to illustrate the dynamics of the plant growth under different treatments.
The effect of PGPR and NPK rates on the leaf area and other plant yield parameters was evaluated for each parameter by using ANOVA. Prior to ANOVA, normality and homoscedasticity of the data were verified using the Ryan-Joiner and Levene tests, respectively (Glèlè Kakaï et al., 2006). Post-hoc or multiple comparison tests (SNK test) of PGPR and NPK doses were performed, and error bars were constructed.
An ascending hierarchical classification with the UPGMA method in the “phangorn” package was performed to identify treatment groups based on the measured parameters. The different classes obtained were described to establish the specific characteristics of each group formed. In addition, a principal component analysis (PCA) was performed to identify the relationships between these parameters and the treatments, and between the treatments and the groups by using the “FactoMineR,” “factoextra,” “corrplot,” and “ggplot2” packages. This procedure was adopted for both PGPR and the combination of PGPR and NPK doses. The analyses were performed in R version 3.6.1 software (R Core Team, 2019).
Results
Soil chemistry of the study site
Table 2 shows the chemical properties of the soil sampled at a depth of 20 cm at the experimental site. The contents of nitrogen (0.6 g/kg), phosphorus (47.5 mg/kg), and carbon (8 g/kg) in the study environment were at average levels and adequate for maize cultivation. The recorded pH value (7.8) indicated that the soil in the study environment was slightly alkaline. The sum of exchangeable bases (7.84 cmol/kg) obtained was also at the mean level in the study site.
Effects of PGPRs on maize plant growth parameters
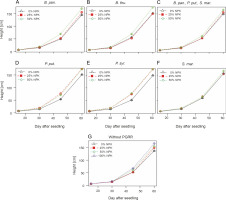
Changes in height of maize plants over time
The best height (166.06 ± 14.75 cm) was obtained for plants fertilized with the recommended dose (100% NPK + urea) (Fig. 2). The ANOVA results indicated a very significant effect (P < 0.001) of fertilization on the height of corn plants. In addition, the combination of PGPR strains and NPK + urea dose (P < 0.05) significantly stimulated the plant height. The most effective treatments were P. put. + 25% NPK + urea and P. syr. + 50% NPK + urea, followed by B. thu. + 50% NPK + urea. These treatments enhanced the plant height to reach 174.17 ± 11.82 cm, 174.25 ± 15.01 cm, and 174.78 ± 21.06 cm, respectively, as compared to 137.39 ± 14.44 cm for the control plants (which received neither PGPR nor NPK).
Changes in diameter of maize plants over time
According to the results of the linear mixed-effects model, the application of the different NPK doses with-out inoculation with PGPRs induced a significant effect (P < 0.0001) on plant crown diameter over time. The curves in Figure 3G indicate that the mean values of the largest collar diameters were recorded with the 100% NPK + urea treatments (1.87 ± 0.13 cm), with an increase of 11.30% compared to that for the control plants. However, the combined effect of PGPR strains and NPK doses on plant crown diameter was not significant (P > 0.05) (Fig. 3). There was a slight increase in the mean diameter in the control and fertilized plants only. Indeed, the diameters of maize plants under the influence of B. pan. + 50% NPK + urea, B. thu. + 50% NPK + urea, P. put. + 50% NPK + urea, P. syr. + 50% NPK + urea, and S. mar. + 50% NPK + urea reached 1.87 ± 0.15 cm, 1.91 ± 0.22 cm, 1.92 ± 0.24 cm, 1.95 ± 0.32 cm, and 1.91 ± 0.22 cm, respectively, as compared to 1.68 ± 0.11 cm for the control plants.
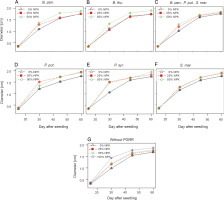
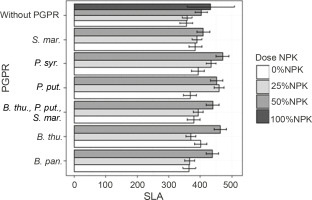
Leaf areas of plants
At 60 DAS, the inoculation treatment with PGPR at various NPK doses induced a very high significant + difference in the plant leaf area compared to the uninoculated controls (P < 0.001) (Fig. 4). Indeed, all leaf area values recorded exceeded those of the control plants (Fig. 4). The highest leaf area values were observed in plants treated with P. syr. + 50% NPK (470.43 cm2), followed by P. put. + 50% NPK (466.38 cm2). Both treatments improved the leaf area up to 32.23 and 31.20%, respectively, as compared to that for the controls.
Combined effects of PGPR and mineral fertilizer doses on the aboveground and belowground plant biomass and their ratios
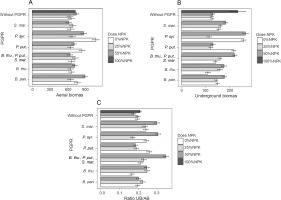
Fresh aerial and underground biomasses and their ratio
The obtained results showed that PGPR inoculation induced better plant development. Indeed, the highest significant value (P < 0.05) of the aboveground fresh biomass was recorded for inoculation with P. syr. (1077.2 g), i.e., an improvement of 67.76% as compared to that of the control plants (Fig. 5A). Two other treatments, namely B. pan. + 50% NPK + urea (896.53 g) and P. syr. + 50% NPK + urea (874.61 g), also stimulated this parameter significantly by 36.21 to 39.62% as compared to that for the controls (Fig. 5A). The lowest fresh aerial biomass was produced by plants inoculated with S. mar. Regarding the fresh underground biomass (Fig. 5B), the two best treatments were P. syr. + 50% NPK + urea and P. syr., which induced an improvement of 109.62 and 103.79%, respectively, compared to that for the controls. Furthermore, the beneficial effect induced by the intake of 100% NPK + urea (86.57%) revealed that the application of P. syr. in the presence of a reduced dose of NPK further stimulated the development of the root system of maize plants. The statistical analysis indicated a very highly significant (P < 0.001) influence of inoculation on the fresh underground biomass of the plants. The values of the fresh biomass ratio (Fig. 5C) recorded with the treatments B. thu., P. put., S. mar. + 50% NPK + urea (84.21%), 100% NPK + urea (63.15%), and P. syr. + 50% NPK + urea (57.89%) showed that the inoculation of PGPR strains with reduced dose of mineral fertilizer was beneficial for the development of maize plants. Factors of interest (NPK dose and PGPR) and their interaction positively influenced the ratio of fresh plant biomass (P < 0.005).
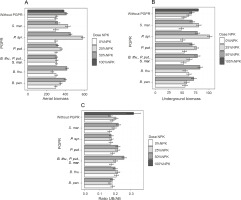
Dry aerial and underground maize biomasses and their ratios
Among the treatments evaluated, two treatments, P. syr. + 25% NPK + urea and P. syr. + 50% NPK + urea, proved to be effective because of their beneficial effect on dry aerial maize biomass. In fact, the dry weights of the underground biomass of the plants were 581.4 ± 36.09 g and 451.7 ± 10.83 g with the application of P. syr. + 25% NPK + urea and P. syr. + 50% NPK + urea, respectively, as compared to 298.9 ± 13.43 g recorded for the control plants (Fig. 6A). In addition, the effect of P. syr. + 25% NPK + urea treatment on the dry weight of the underground biomass of the plants was also better. The results (Fig. 6B) showed that the greatest production of dry underground biomass was obtained with this treatment, i.e., an improvement of 89.09% more than that for the control plants. The ANOVA results indicated that the combination of PGPR and mineral fertilizer dose had a highly significant effect on biomass dry weight (P < 0.001). Similarly, the effects of the PGPR factor and the combination of PGPR and NPK dose were significant on the ratio of plant dry biomass. The highest ratio was obtained when the full dose (100% NPK + urea) was used to fertilize the plants.
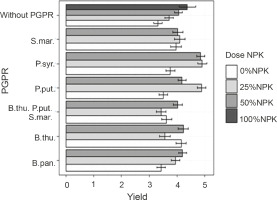
Effects of PGPRs and the combination of different NPK doses on maize grain yield
Figure 7 shows the effect of PGPR inoculation based on mineral fertilizer doses on grain yield. The best yields were recorded with the treatments P. syr. + 25% NPK + urea, P. syr. + 50% NPK + urea, and P. put. + 25% NPK + urea. However, no significant difference was observed between the effects induced by these treatments. Their applications increased the yields by 30.64 to 32.25% as compared to that of the control plants. For the plants fertilized with 100% NPK + urea, a lower grain yield was recorded compared to that for plants inoculated with P. syringae and P. putida in the presence of reduced doses of NPK + urea, with an improvement rate of 17.74% as compared to that for the controls. The ANOVA results showed a significantly positive influence (P < 0.001) of the combined application of PGPR strain inoculation and reduced NPK doses on grain yield.
Classification of treatments based on the performance of PGPR strains tested in the absence of NPK
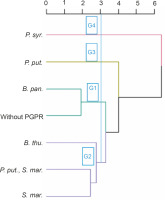
Four groups of PGPRs emerged from the bottom-up hierarchical classification. These groups (G1, G2, G3, and G4) included 2 (B. pan. and without PGPR), 3 (S. mar., B. thu., P. put., B. thu.), 1 (P. put.), and 1 (P. syr.) treatments, respectively (Fig. 8). The results of ANOVA applied within the classes showed that these identified homogeneous groups were significantly different (P < 0.001) from each other in terms of the different parameters evaluated. Groups 3 and 4 showed the best performance for all the parameters tested.
Fig. 8
Dendrogram showing hierarchical classification of treatments based on the performance of PGPR strains tested in the absence of NPK. B. thu. – Bacillus thuringiensis, B. pan. – Bacillus panthothenicus, P. put. – Pseudomonas putida, P. syr. – Pseudomonas syringae, S. mar. – Serratia marcescens
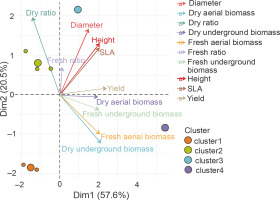
A Main Component Analysis (CPA) was conducted to establish the correlation between the parameters assessed in the homogeneous groups and to identify the best treatments (Fig. 9). The results showed that all the parameters measured were positively correlated with axis 1, except for the UB/AB ratio. The latter was positively correlated with the second axis. Axis 1 therefore considers all the measured parameters. The projection of the groups and parameters on the first two axes (Fig. 9) confirmed the description of the classes obtained.
Fig. 9
Principal Component Analysis (PCA) of the relationship between height, diameter, leaf area (SLA), dry ratio, dry underground biomass, dry aerial biomass, fresh aerial biomass, yield, fresh ratio, and treatment groups G1 (cluster 1), G2 (cluster 2), G3 (cluster 3), G4 (cluster 4), and G5 (cluster 5) – PGPR inoculation without NPK fertilizer
Classification of maize treatments on the basis of the performance of PGPR strains combined with different doses of NPK
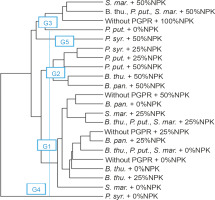
The second level of classification considered all the treatments compared. The dendrogram showed that the treatments were divided into five homogeneous groups. These groups (G4, G3, G5, G2, and G1) included 1, 4, 1, 5, and 11 treatments, respectively (Fig. 10).
Fig. 10
Dendrogram showing hierarchical classification of treatments on the basis of the performance of PGPR strains combined with different doses of NPK. B. thu. – Bacillus thuringiensis, B. pan. – Bacillus panthothenicus, P. put. – Pseudomonas putida, P. syr. – Pseudomonas syringae, S. mar. – Serratia marcescens
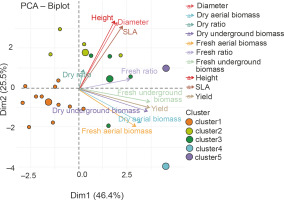
The ANOVA results indicated the best performance of groups G2 (P. put. + 50% NPK + urea, P. put. + 25% NPK + urea, and P. syr. + 25% NPK + urea) and G5 (P. syr. + 50% NPK + urea) on maize plant growth, biomass, and grain yield parameters. Figure 11 shows the correlation between the parameters evaluated within the groups. The analysis of this figure showed that parameters such as yield and biomass are correlated with axis 1, while growth parameters are correlated with axis 2. This projection of the groups and parameters on the first two axes allowed to select P. syr. + 50% NPK + urea as the most discriminating treatment.
Fig. 11
Principal Component Analysis (PCA) of the relationship between height, diameter, leaf area (SLA), dry ratio, dry underground biomass, dry aerial biomass, fresh aerial biomass, yield, fresh ratio, and treatment groups G1 (cluster 1), G2 (cluster 2), G3 (cluster 3), G4 (cluster 4), and G5 (cluster 5) – PGPR inoculation combined with NPK fertilizer – from hierarchical classification
Discussion
The use of PGPR strains in agricultural practices is strongly encouraged, as they can serve as a sustainable solution for improving the effectiveness of chemical fertilizers. The growth and development of different crops under various environmental conditions have been reported in response to PGPR inoculation with or without mineral fertilizers (Yao et al., 2006; Krey et al., 2013; Verma et al., 2013; Zahid et al., 2015; Pereira et al., 2020). In the present study, a positive effect of PGPR inoculation was noted on the height, diameter, biomass, and grain yield of maize plants with the intake of NPK mineral fertilizer. These results are similar to those reported by Saber et al. (2012) and Lavakush et al. (2014) who stated that the application of PGPRs as biological inoculants increases the growth and grain yield of cereals. In a recent study conducted in India, Zafar-ul-Hye et al. (2018) reported the efficacy of P. syringae + 50% NPK + urea on maize plant height; an improvement of 63.63% and 119.5%, respectively, was observed compared to the height of maize plants grown under the influence of recommended fertilizer application and control plants. In Northern Benin, the application of P. syr. + 50% NPK + urea at the time of sowing maize of the 2000 SYNEE variety (an extra-precocious variety with a vegetative cycle of 80 days, a potential yield of 4 t/ha on the research station and 2.5 t/ha in the farming environment) showed positive results, resulting in again of 35.72% and 23.77% on the height and diameter, respectively, measured at the collar of maize plants (Amogou et al., 2019). In the present study, the height of plants under the effect of P. syr. + 50% NPK + urea improved by 26.82% compared to that for the control plants. Although the response to inoculation with halfdose mineral fertilizer was highly variable, the effectiveness of P. syr. + 50% NPK + urea was greater among all the treatments on the leaf surface when compared with the controls. On the other hand, the combined effect of inoculation and fertilization on the plant collar diameter was not significant. However, a slight improvement in the performance of P. syr. + 50% NPK + urea was noted for this parameter (4.27 and 16.38% increase as compared to that for 100% NPK + urea application and controls, respectively).
The production of fresh aerial underground biomass was significantly improved compared not only to the control plants but also to fertilization in response to inoculation of maize plants by P. syringae. Indeed, with the application of P. syr. + 50% NPK + urea and P. syr. alone, the root system of the plants was more improved (109.62 and 103.79%, respectively) as compared to that for the controls. This performance exceeded the beneficial effect induced by the intake of 100% NPK + urea (86.57%), indicating that the use of this bacterial strain in the presence of a reduced dose of NPK would be more appropriate for sustainable maize production in central Benin. Our results corroborate those reported by Isfahani et al. (2012) who showed an improvement in root dry weight of treated cucumber as compared to that of controls, while the lowest dry weight was recorded in plants that received the full dose of 100% NPK mineral fertilizer (P100B0). Zafar-ul-Hye et al. (2015) reported that the application of P. syringae in the presence of 50% NPK + urea on maize seeds resulted in better improvement of fresh and dry underground biomass of the plants (55.54 and 57.83%, respectively) as compared to those of unfertilized and uninoculated plants, respectively. According to these authors, the effectiveness of P. syringae could be explained by its ability to synthesize ACC deaminase, an enzyme known to be involved in reducing the harmful effects of ethylene produced by plants in response to saline stress. In addition, Ambrosini and Passaglia (2017) linked the promotion of plant growth to the ability of rhizobacteria to increase the activity of 1-aminocyclopropane-1-carboxylate deaminase. In terms of grain yield recorded in the present study, the best productivity was recorded with P. syr. + 25% NKP + urea, P. syr. + 50% NPK + urea, and P. put. + 25% NPK + urea. However, no significant difference was observed between the effects induced by these treatments. Their applications increased the yield by 30.64 to 32.25% as compared to that of the control plants. Our results were better than those reported by Amogou et al. (2019) who found 14.20% increase in the yield for maize seeds treated with P. syringae inoculation + 50% NPK + urea. Moreover, these authors showed that P. put. + 50% NPK + urea resulted in an increase in grain yield (35.5%) as compared to that for the controls. These results are similar to those obtained with P. put. + 25% NPK + urea in our study. Although both these studies were conducted on the same type of soil (ferruginous soil), the difference in the performance of P. syr. + 50% NPK + urea might be related to the type of maize variety used. Lucy et al. (2004) showed that the effectiveness of PGPR strains varies considerably depending on the environmental conditions under study and that their ability to promote plant growth and development can be influenced by specific plant species, cultivars, soil type, and genotype. In another study on maize conducted by Sood et al. (2018), co-inoculation of a PGPR (B1N1) with 80% of the recommended rate of NP mineral fertilizer significantly increased maize grain yield by 11.7% as compared to that achieved after the application of the full recommended rate of mineral fertilizer (100% NP). A 50% reduction in the use of NPK fertilizers and an improvement in grain yield of maize and barley have also been reported in other studies (Mehrvarz et al., 2008; Yazdani et al., 2009). The mechanisms by which the investigated PGPR strains promote plant growth were not evaluated in our study. Despite the reduction in the dose of mineral fertilizer, the good increase in all growth and yield parameters of plants inoculated with P. syr. + 50% NPK + urea can be explained by several factors. The P. syringae strain is among those reported PGPR isolates with multiple attributes that can significantly influence crop growth and yield (Abgodjato et al., 2018). PGPRs can promote plant growth by using their own metabolic activities (phosphate solubilization, hormone production, or nitrogen xation), by directly a ecting plant metabolism (increased water and mineral uptake), by improving root development, by increasing plant enzymatic activity, by “helping” other bene cial microorganisms to enhance their effect on plants, or by suppressing plant pathogens (Mantelin and Touraine, 2004; Pérez-Montaño et al., 2014). Several researchers (Bashan et al., 2004; Labidi et al., 2016) have explained the positive effect of PGPRs on the basis of their ability to produce auxins, represented primarily by indole acetic acid (IAA). The latter promotes greater capacity to absorb nutrients (Cassán and Diaz-Zorita, 2016) and the accumulation of reserves, which increases the diameter of the stems (Dartora et al., 2013), thus contributing to greater plant development. Fancelli and Dourado Neto (2000) reported that the larger the diameter of maize plants, the better these plants are able to resist the increasingly difficult environmental conditions, which contributes to a greater accumulation of organic solutes in the plants. This leads to better grain filling and therefore higher yields (Kappes et al., 2011).
Conclusion
The results of the present study confirmed the efficacy of PGPR inoculation in combination with reduced rates of mineral fertilizer on maize growth and yield parameters. In the presence of 50% of the recommended dose of mineral fertilizer, the inoculation of P. syringae resulted in best improvements in height, leaf area, biomass, and grain yield of maize. Thus PGPR should be evaluated in large field trials to assess their potential for use as biofertilizers.