Introduction
The fungal genus Ganoderma has long been used as a traditional medicinal herb in East Asia (Luangharn et al., 2021). Because the morphologies of Ganoderma species are similar, their taxonomies remain unclear (Yalcin et al., 2020). Approximately 400 species have been described in this genus. Ganoderma sinense, commonly known as “Lingzhi,” is distributed in subtropical–tropical regions (Hapuarachchi, 2019a). Similar to other species of the genus Ganoderma, G. sinense is used as herbal medicine and health supplement. It has been reported to have antitumor (Han et al., 2021), antiaging (Teseo et al., 2021), anti-inflammatory (Mei et al., 2019), and anticancer (Zheng et al., 2018) activities and nitric oxide inhibitory properties (Wang et al., 2020). Polysaccharides are the major biological components of G. sinense (Y. Zhang et al., 2019).
One of the most significant challenges in the mushroom cultivation industry is identifying superior mushrooms with high yields and high stress tolerance. The cultivation of wild strains before their extinction is considered a realistic approach to overcome this challenge. Previous studies have reported the successful cultivation of several wild mushroom strains, such as Lepista sordida (Thongbai et al., 2017), Trametes versicolor (Nguyen et al., 2021), and Pycnoporus sanguineus (Dulay and Damaso, 2020). However, to the best of our knowledge, no study has evaluated the potential cultivation of wild G. sinense strains.
Culture conditions are the primary factors affecting the mycelial growth and fruiting body formation in mushrooms (Nguyen et al., 2021). Among Ganoderma species, the majority of the studies focused on identifying the culture conditions for the mycelial growth of Ganoderma lucidum (Jayasinghe et al., 2008) and Ganoderma applanatum (Jo et al., 2009), but not for other species. Reportedly, the optimal culture conditions for the mycelial growth of G. lucidum are the yeast malt extract medium (YM) at pH 5 and 25–30°C (Jayasinghe et al., 2008), whereas G. applanatum grows well on potato dextrose agar (PDA), yeast mannitol agar, and mushroom complete medium at 25–30°C (Jo et al., 2009).
As Lingzhi mushrooms are scarce in nature, they are artificially cultivated to meet the increasing demands of international markets. Due to the abundance of available agro-industrial waste and human resources, there are good prospects for growing Ganoderma in Vietnam. The Lingzhi mushroom cultivation industry improves sustainable rural development in Vietnam (Ngo et al., 2019). Various methods such as log cultivation, sawdust cultivation, and substituted cultivation have been developed to cultivate Lingzhi mushrooms (Zhou et al., 2012), with sawdust, corncobs, cottonseed husk, and bagasse being the primary substrates (Zhou, 2017). Little is known about the optimal conditions required for mycelial growth and fruiting body formation in G. sinense.
In this study, a wild G. sinense strain collected from Lang Son Province was isolated, and the culture conditions for its mycelial growth and fruiting body formation were optimized. This study may provide useful information about spawn production and cultivation of wild G. sinense strains.
Material and methods
Fungal strains
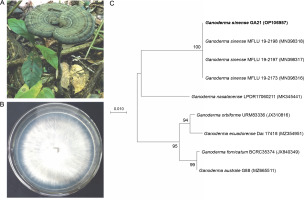
Strain GA21 was collected from Lang Son Province in northern Vietnam (GPS location: 21°39′57″N 107°07′45″E) – Figure 1. The voucher specimen (no. GA21) was deposited at the Mushroom Research and Development Center, Vietnam. Pure mycelial cultures were isolated from healthy fruiting bodies using a previously described tissue culture technique (Nguyen et al., 2021). The inner tissue (approximately 3 cm) of the fruiting body was inoculated in a PDA medium in a Petri dish at 25°C for 7 days in a dark room. The mycelium was maintained on PDA slants at 4°C for further studies. To identify strain GA21, the internal transcribed spacer (ITS) was amplified and sequenced using the universal primer set ITS5 (5′GGA AGT AAA AGT CGT AAC AAG G3′) and ITS4 (5′TCC TCC GCT TAT TGA TAT GC3′) (White et al., 1990). The ITS sequence of strain GA21 was deposited in GenBank under the accession number OP106957. In addition, the ITS regions of G. sinense MFLU 19-2198, G. sinense 19-2197, G. sinense 19-2173, Ganoderma nasalanense LPDR 17060211, Ganoderma orbiforme URM83336, Ganoderma ecuadorense Dai 17418, Ganoderma fornicatum BCRC35374, and Ganoderma australe G88 were retrieved from GenBank. Phylogenetic trees were constructed using maximum-likelihood algorithms (Felsenstein, 1981) in the Molecular Evolutionary Genetics Analysis (MEGA) X software (Kumar et al., 2018). The robustness of the phylogenetic trees was evaluated using a bootstrap procedure based on 1000 replications.
Fig. 1
Morphological features of Ganoderma sinense strain GA21: mature basidiomes (A), mycelium grown on potato dextrose agar medium (B); maximum-likelihood phylogenetic tree based on internal transcribed spacer sequences showing the relationship of strain GA21 to other members of the genus Ganoderma
Nutrition effects on mycelial growth
The optimal nutritional requirements for the growth of G. sinense were identified by examining one factor at a time. The basal medium was the potato–agar medium containing 200 g/l infused potato and 15 g/l agar powder (Hailong Co Ltd, Vietnam). Potato infusion was prepared as described previously (Nguyen et al., 2019). Carbon sources were tested at a concentration of 20 g/l, which included glucose, lactose, saccharose, starch, fructose, maltose, xylose, and dextrin (Merk, Germany). Based on the obtained results, fructose was selected as the optimal carbon source. To optimize the concentration of the carbon source, fructose was enriched into the basal medium at multiple concentrations (0, 10, 15, 20, 25, and 30 g/l). Similarly, G. sinense was inoculated in a basal medium supplemented with various nitrogen sources (ammonium chloride [NH4Cl], ammonium nitrate [NH4NO3], ammonium sulfate [(NH4)2SO4], peptone, yeast powder, and urea) (Merk, Germany). The yeast extract was evaluated at various concentrations (0, 1, 2, 3, 4, and 5 g/l) as the best nitrogen source for the mycelial growth of strain GA21.
Effects of temperature and pH on mycelial growth
To optimize the culture conditions in terms of temperature, mycelium disks of G. sinense (approximately 1 cm diameter) were inoculated on PDA plates and incubated at different temperatures (15, 20, 25, 30, and 35°C). In addition, eight pH values (pH 3, 4, 5, 6, 7, 8, 9, and 10) were evaluated to identify the optimal pH for the favorable growth of G. sinense. The pH of the growth media was adjusted using 0.1 M NaOH or 0.1 M HCl.
Grain spawn preparation
To upscale the mycelium of strain GA21, rice grains and rubber wood sawdust were selected as spawn substrates. Rice grains and sawdust (Thanh Cao KINOKO I.E Co., Ltd) were prepared according to previously reported protocols (Nguyen et al., 2019). In brief, rice grains were washed and soaked in water overnight. The grains were boiled for 20–25 min until they became soft. Rubber wood sawdust was mixed with a lime solution (0.4%) and fermented for 5–7 days. Then, the moisture level of the substrates was adjusted to 65% using tap water. The following compositions of the culture media were used: treatment I (99% rice grains + 1% calcium carbonate), treatment II (69% rice grains + 30% sawdust + 1% calcium carbonate), and treatment III (39% rice grains + 60% sawdust + 1% calcium carbonate). The mixtures were filled into 24-ml round-bottom tubes to their three-fourth capacity and sterilized at 121°C for 60 min. As soon as the culture media reached room temperature, the sterilized tubes were inoculated with a piece of mycelia culture (5 mm × 5 mm) and incubated for 20 days at 25 ± 1°C under dark conditions.
Substrate preparation and cultivation
In the cultivation of G. sinense, rubber wood sawdust was selected as the primary substrate medium. Treatments with various combinations of sawdust and wheat bran (Thanh Cao KINOKO I.E Co., Ltd) were used to cultivate G. sinense : treatment A (99% sawdust, 0% wheat bran, 1% calcium carbonate), treatment B (98% sawdust, 1% wheat bran, 1% calcium carbonate), and treatment C (96% sawdust, 3% wheat bran, 1% calcium carbonate).
Polythene bags were filled with 1.2 kg of the substrates and autoclaved at 121°C for 90 min. After cooling, the substrates were inoculated with the master spawn in 5% of substrate wet weight. The inoculated bags were moved to the spawn running room and incubated at 25°C with an air relative humidity of 65% under dark conditions until complete colonization of the substrate (20 days). The bags were incubated at 25°C and 85% air relative humidity in the cultivation room to induce fruiting body formation.
Data collection
The morphology of G. sinense mycelium was characterized based on the growth rate (mm/day), density [high, 3+; regular, 2+; and low, 1+], texture (cottony and floccose), and radial growth distribution (uniform and uneven) of mycelium. The mycelial growth rate (mm/day) was calculated after 8 days of incubation.
The growth performance of G. sinense was determined based on the following cultivation parameters: spawn running period, pinhead formation, and fruiting body development. The spawn running period (days) was the period required for G. sinense to achieve complete colonization of the substrate. The day of pinhead formation (days) was considered the day of inoculation to the primordia. The biological efficiency (BE, in %) was calculated based on the ratio of the dry fruiting body weight (g) to the dry weight of the substrate (g). To calculate dry weight, the fruiting bodies of G. sinense were dried at 60°C for 24 h.
Statistical methods
Data were analyzed using GraphPad Prism (version 9.0, GraphPad Software Inc., San Diego, CA). All experiments were conducted in triplicate. The obtained data were subjected to one-way Analysis of variance (ANOVA), followed by Tukey’s multiple-range tests (P < 0.05), which were represented by letters or asterisks.
Results and discussion
Mycelial morphology of strain GA21
The naturally occurring pileus was kidney shaped, and the color of strain GA21 was dark brown (Fig. 1A). The pileus size of strain GA21 was 7.9 cm in length, 10.8 cm in width, and up to 2.5 cm in thickness at the base (Fig. 1A). This strain grows well on the PDA medium. The mycelial morphology of strain GA21 was cottony with white pigmentation and uniform radial growth (Fig. 1B). Strain GA21 showed the highest ITS sequence similarity of 99.82% with G. sinense MFLU 19-2198, G. sinense MFLU 19-2197, G. sinense MFLU 19-2173, and G. sinense GACP171012239. In the phylogenetic tree, strain GA21 formed a robust cluster with G. sinense MFLU 19-2198, G. sinense MFLU 19-2197, and G. sinense MFLU 19-2173, with a high bootstrap support value of 100% (Fig. 1C).
G. sinense has high phenotypic plasticity (Hapuarachchi, 2019b), with its morphology varying depending on climate, nutrition, and geographical environment (Hapuarachchi, 2019b). The color of G. sinense fruiting bodies was yellowish brown, brownish orange, gray, brownish black, and dark brown (Hapuarachchi, 2019b). The morphological characteristics of strain GA21 were similar to those of G. sinense. However, to further confirm the taxonomic position of strain GA21, the ITS region was amplified and sequenced using the universal primer set ITS5 and ITS4. ITS is an informative DNA region used for the classification of fungi (Schoch et al., 2012). The ITS sequence similarity and the phylogenetic tree confirmed that strain GA21 belongs to G. sinense. To the best of our knowledge, this is the first report of the occurrence of G. sinense in Vietnam.
Effect of carbon sources on mycelial growth
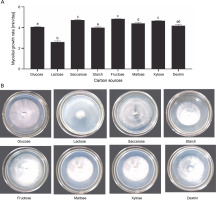
The culture medium is a vital intrinsic factor since it provides essential nutrients for mycelium growth (Ta et al., 2021). The nutrition requirements for the mycelium growth of Lingzhi mushrooms primarily include carbon and nitrogen resources (Zhou et al., 2012). Carbon sources, which act as structural and storage compounds in the cell, should be optimized first among the constituents of nutritional compositions (Nguyen et al., 2021). G. lucidum can use a wide range of carbon sources, including dextrin, galactose, mannose, maltose, sucrose, and xylose (Jayasinghe et al., 2008). Of the eight carbon sources tested in this study, G. sinense grew well on the medium supplemented with fructose (4.82 ± 0.05 mm/day), saccharose (4.71 ± 0.41 mm/day), and maltose (4.40 ± 0.14 mm/day) (Fig. 2A). In contrast, low growth was observed in the medium enriched with lactose, with a mycelial growth rate of 2.60 ± 0.20 mm/day (Fig. 2A). In addition, fructose treatment showed a higher mycelial density (3+) than the other carbon sources (Fig. 2B). Therefore, fructose was considered the best carbon source for mycelial growth.
Fig. 2
Effect of carbon sources on the mycelial growth rate (A) and mycelial morphology of Ganoderma sinense on day 8 (B); different letters denote statistically significant differences at P < 0.05
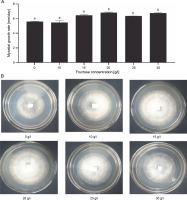
To evaluate the effects of the concentration of the carbon source on the growth rate of G. sinense, fructose was supplied into the medium at different concentrations (10, 15, 20, 25, and 30 g/l), along with a medium devoid of fructose. The carbon content had a significant effect on the mycelial growth of G. sinense. The lowest mycelial diameter (5.45–5.60 mm/day) was observed in the medium containing 0–10 g/l of fructose (Fig. 3A). The highest mycelial growth was observed with 15 g/l (6.45 ± 0.14 mm/day), 20 g/l (6.82 ± 0.10 mm/day), and 30 g/l (6.75 ± 0.10 mm/day) fructose treatments. Thus, fructose with a carbon content of 15 g/l was selected for further experiments.
Effect of nitrogen sources on mycelial growth
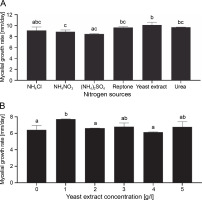
Mushrooms require nitrogen for the synthesis of nitrogen-containing compounds and chitin in the cell wall (Miles and Chang, 1997). They can use both inorganic (salts of nitrate, ammonium ions, etc.) and organic nitrogen sources (peptone, yeast extract, etc.) (Miles and Chang, 1997). Importantly, under nitrogen-deficient conditions, mycelium might stop growing. Alternatively, a high nitrogen content may inhibit mycelium growth due to the formation of metabolic byproducts (Junior Letti et al., 2018). The most suitable nitrogen sources for the mycelial growth of G. lucidum are ammonium acetate, glycine, arginine, and calcium nitrate (Jayasinghe et al., 2008). In the present study, three organic nitrogen sources (peptone, yeast extract, and urea) and three inorganic nitrogen sources [NH4Cl, NH4NO3, and (NH4)2SO4] at a concentration of 5 g/l were used to optimize the culture conditions of G. sinense. Results showed that G. sinense grow on the media supplemented with all tested nitrogen sources (Fig. 4A). The medium supplemented with yeast extract showed a higher growth rate (10.11 mm/day) than other nitrogen sources. Furthermore, the highest mycelial density (3+) was observed for yeast extract and peptone. Therefore, yeast extract was selected as an optimal nitrogen source for the mycelial growth of G. sinense as it grew best on a medium containing 1 g/l yeast extract (7.68 ± 0.06 mm/day) (Fig. 4B).
Effect of temperature on mycelial growth
In mushrooms, temperature affects nitrogen and sugar assimilation, respiration, and biosynthesis (Ta et al., 2021). This abiotic factor is one of the most significant physical factors affecting the mycelial growth and development of fruiting bodies (Nguyen et al., 2019). Different species require different temperatures for mycelial growth. For example, the most favorable temperatures for the mycelial growth of G. lucidum, Laetiporus sulphureus, Agaricus bisporus, and Calocybe indica were 30°C (Nguyen et al., 2019), 25–30°C (Luangharn et al., 2014), 22–24°C (Ma et al., 2014), and 30–32°C (Min et al., 2020), respectively. For G. sinense, the mycelial growth rate was the highest at 25°C (10.86 ± 0.04 mm/day) and 30°C (10.85 ± 0.02 mm/day), followed by 20°C (8.72 ± 0.15 mm/day) (Fig. 5A). No mycelial growth occurred at 35°C. Basidiomycetes can be divided into three groups based on their optimal temperature range for mycelial growth: low temperature (optimum temperature: 25–30°C), intermediate (optimum temperature: 30–37°C), and high temperature (optimum temperature: 37–40°C) (Mswaka and Magan, 1999). For the low-temperature group, the ideal temperatures for mycelial growth ranged from 25 to 30°C, and they did not grow at temperatures above 37°C. Therefore, G. sinense was considered to belong to the low-temperature basidiomycetes group.
Fig. 5
Effect of temperature (A) and pH (B) on the mycelial growth rate of Ganoderma sinense; different letters denote statistically significant differences at P < 0.05
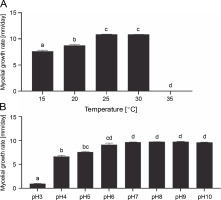
The mycelial density was thick (3+) at 25°C but somewhat thick (2+) at 20 and 30°C. The mycelial texture of strain GA21 was cottony, and its pigmentation was white. Taken together, G. sinense grew most efficiently at 25°C.
Effect of pH on mycelial growth
pH has a significant impact on cell membrane function, the absorption of nutrients, cell morphology, enzyme activity, and product biosynthesis (Ta et al., 2021). G. sinense grew at a pH range of 3.0–10.0 (Fig. 5B). This finding is consistent with those of previous studies showing that Ganoderma species can grow in a wide pH range (Jayasinghe et al., 2008; Nguyen et al., 2019). The optimal pH for enhancing Ganoderma mycelial growth varies from one species to another. For example, the maximum mycelial growth of G. lucidum was observed at pH 4.5–5.5 (Jayasinghe et al., 2008; Subedi et al., 2021), whereas Ganoderma resinaceum and G. australe grew fastest at pH 7 and pH 7–8, respectively (Kim et al., 2006; Luangharn et al., 2017). For the growth of G. sinense, pH 7 proved to be optimal (Fig. 5B). At this pH level, the mycelial growth rate was 9.63 mm/day, and the density was high (3+).
Mycelial growth on spawn substrates
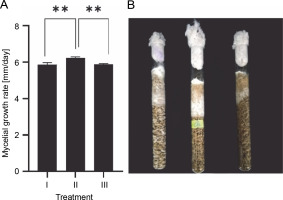
Spawn plays an important role in determining the biological efficiency of mushrooms. Using grains (e.g., wheat, barley, sorghum, brown rice) in spawn production causes high contamination rates due to their high nutrition (W.R. Zhang et al., 2019). Furthermore, the use of grains increases manufacturing costs (W.R. Zhang et al., 2019). Using a mixture of sawdust and rice is an effective method for reducing production costs and contamination rates (Nguyen et al., 2021). For example, the optimal substrate for T. versicolor spawn production is a mixture of 20% rice grains, 79% sawdust, and 1% calcium carbonate (Nguyen et al., 2021). In the present study, sawdust was mixed with rice at different ratios and used as spawn substrates. Spawn grain substrates had a significant influence on the mycelial growth rate of G. sinense (Fig. 6). G. sinense began to colonize spawn substrates on day 2. Treatment II showed the highest fungal growth rate (6.23 ± 0.06 mm/day), followed by treatment III (5.9 ± 0.11 mm/day) and treatment I (5.8 ± 0.03 mm/day). A higher mycelial density was observed in treatments I and II than in treatment III (Fig. 6B). In brief, treatment II (69% rice grains + 30% sawdust + 1% calcium carbonate) was the most suitable culture media for the mycelial growth of G. sinense.
Fig. 6
Effect of various spawning materials on the mycelial growth rate (A) and density (B) of Ganoderma sinense; treatment I (99% rice grains + 1% calcium carbonate), treatment II (69% rice grains + 30% sawdust + 1% calcium carbonate), and treatment III (39% rice grains + 60% sawdust + 1% calcium carbonate); Asterisks show significant differences (**P < 0.01)
Cultivation characteristics
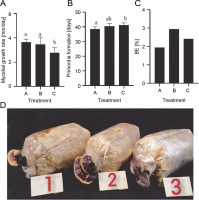
Due to its medicinal and cultural importance, lingzhi has huge economic value (Bishop et al., 2015). Temperature, light, and nutrient availability play crucial roles in mushroom fructification. Under warm conditions, lingzhi mushrooms may develop fruiting bodies earlier (Bijalwan et al., 2021). The yield performance and quality of the mushrooms are strongly dependent on substrate cultivation. The substrates used in mushroom cultivation are generally selected based on locally available waste. In this study, sawdust was used as a basal substrate. Since sawdust has a low nutrient content and may be insufficient for the development of fruiting bodies, it was enriched with wheat bran, which acts as a nitrogen reservoir for mushrooms (Nguyen et al., 2021). However, high supplementation with wheat bran can increase the risk of contamination, inhibit the mycelial growth, and reduce the yield of mushrooms (Nguyen et al., 2021). The highest mycelial growth rate of G. sinense was observed in treatment A (99% sawdust, 1% calcium carbonate) (3.65 ± 0.24 mm/day) and treatment B (98% sawdust, 1% wheat bran, 1% calcium carbonate) (3.47 ± 0.60 mm/day). In contrast, compared with treatments B and A, treatment C (96% sawdust, 3% wheat bran, 1% calcium carbonate) showed a lower mycelial growth rate (2.76 ± 0.43 mm/day).
Ganoderma species can be artificially cultivated using many substrates, such as sawdust, tea waste, cotton seed husk, corn cobs, and sunflower seed hulls (Hapuarachchi et al., 2018). All analyzed treatments induced the primordia formation of G. sinense (Fig. 7B), suggesting the possibility of G. sinense cultivation using rubber wood sawdust. In G. sinense, primordia formation was observed on the 38th day in treatment A, the 40th day in treatment B, and the 41st day in treatment C (Fig. 7B). Under artificial cultural conditions, G. lucidum forms primordia after inoculation for 43–48 days (Ngo et al., 2019). Therefore, the cropping cycle of G. sinense was similar to that of G. lucidum. A previous study reported that the yield of G. lucidum was increased when grown on a cultivation substrate supplemented with wheat bran (Mehta et al., 2014). Similarly, in the present study, the wheat bran content was found to affect the yield of G. sinense. The highest BE was achieved in treatment B (3.0%), followed by treatment A (2.4%) and treatment C (1.9%) (Fig. 7C and D). Thus, treatment B (98% sawdust, 1% wheat bran, 1% calcium carbonate) can be considered the optimal substrate mixture for G. sinense cultivation.
Fig. 7
Effect of different cultivation substrates on the mycelial growth rate (A), primordia formation (B), and BE (C) of Ganoderma sinense; treatment A (99% sawdust, 0% wheat bran, 1% calcium carbonate), treatment B (98% sawdust, 1% wheat bran, 1% calcium carbonate), and treatment C (96% sawdust, 3% wheat bran, 1% calcium carbonate); different letters denote statistically significant differences at P < 0.05
Conclusions
Despite being a well-known medicinal macrofungus, little is known about the optimal cultivation conditions of G. sinense. In this study, the optimal cultural conditions for the mycelial growth of G. sinense were as follows: fructose (15 g/l), yeast extract (1 g/l), pH 7, and 25–30°C. This study demonstrated for the first time that G. sinense can form primordia and develop fruiting bodies on sawdust as the base substrate. Sawdust enriched with 1% wheat bran could be a suitable and inexpensive substrate for G. sinense cultivation. The findings of this study contribute to the industrialization of G. sinense cultivation. Further studies are needed to optimize the temperature and humidity for G. sinense cultivation and assess its medicinal value.