Introduction
Multidrug resistance (MDR) is a phenomenon in which microorganisms develop resistance to drugs, even if they were once sensitive to them. This resistance contributes to increased disease and mortality rates (Tanwar et al., 2014). The search for new medications to treat health issues and infections is crucial, particularly in light of the global issue of antibiotic resistance. The impact of antibiotic resistance has been significant, with an expected 5.2 million people in the Western Pacific Region projected to succumb to drug-resistant bacterial infections by the end of 2030 (WHO, 2023). According to Pasrija et al. (2022), the percentage of infections caused by resistant microorganisms is 18% in Southeast Asia, 12% in the Western Pacific region, and 6% in Africa. Additionally, it is estimated that by 2050, MDR infections will lead to approximately 10 million deaths annually (Tagliaferri et al., 2020).
Endophytic fungi, residing in plant tissues without causing visible disease symptoms, have garnered significant attention for their ability to produce various secondary metabolites (Manganyi and Ateba, 2020; Mamangkey et al., 2022a). The natural substances produced by fungal endophytes exhibit a range of biological characteristics, including antimicrobial capabilities (Joo et al., 2021; Mamangkey et al., 2022b). Endophytes have been isolated from all studied plant species, and it is recognized that, among the estimated 300 000 known plant species on Earth, each hosts at least one endophytic resident. However, only a small fraction of plant species (approximately 1–2%) have been investigated for their endophytic populations (Strobel, 2018). Endophytes are located in various parts of the plant host, including single cells along vascular tissues and clusters inside certain epidermal cells, with uneven distribution within host tissue (de Souza et al., 2004). The need for an alternative to chemosynthetic medications for treating human diseases has grown, highlighting the importance of bioactive molecules produced by endophytes to combat the advent of new drug-resistant infections (Kraupner et al., 2018; Bengtsson-Palme et al., 2018; Adeleke and Babalola, 2020).
Common endophytic fungal species, particularly those belonging to the genera Aspergillus and Penicillium, are recognized as primary producers of certain toxic substances known as mycotoxins (Peilu et al., 2018). Despite this, these fungi also serve as abundant sources of bioactive compounds with potential medicinal benefits, as highlighted by George et al. (2019). Therefore, this study explores the different bioactive substances derived from endophytic fungi within the Aspergillii and Penicillii, particularly in conjunction with medicinal plants. The emphasis is on their significance in microbiology, including their antimicrobial activity and their ability to inhibit multidrug-resistant pathogens.
Bioactive compounds
An antimicrobial is a substance capable of killing or inhibiting the growth of microorganisms, such as bacteria and fungi, thereby preventing their proliferation. Many researchers are currently investigating various extracts of endophytic fungi as potential antimicrobial agents. Filamentous fungi, including Aspergillus and Penicillium, which contribute to nearly 99% of all known fungal metabolites, are recognized for producing approximately 45% of known microbial metabolites (Berdy, 2012). This positions them as a particularly promising source of bioactive compounds for antimicrobial purposes. Endophytic fungi are known to synthesize a wide variety of chemicals, categorized into groups such as aliphatic metabolites, alkaloids, flavonoids, glycosides, lactones, phenyl propanoids, quinones, steroids, terpenoids, and xanthones (Zhang et al., 2006).
Aspergillus stands out as one of the most widely distributed genera of endophytic fungi, capable of forming symbiotic relationships with various organisms, including plants. Endophytic Aspergillus species have been shown to synthesize diverse secondary metabolites, including butenolides, alkaloids, terpenoids, cytochalasins, phenalenones, terphenyls, xanthones, sterols, diphenyl ether, and anthraquinones. These compounds hold substantial significance for the pharmaceutical and commercial sectors (El-hawary et al., 2020), making Aspergillus a valuable reservoir of bioactive molecules for medical applications. Aspergillus members are also gaining recognition as prolific producers of bioactive compounds, particularly antimicrobial agents. According to Domingos et al. (2022), Aspergillus is considered to possess the highest bioactive potential among all ascomycete fungi in the natural world. Extensive research has been conducted on species within this genus, known for generating metabolites of considerable economic and medicinal importance.
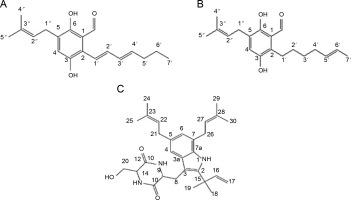
The species Aspergillus versicolor has been reported to produce aniduquinolone A (Fig. 1A), an antibacterial compound inhibiting the growth of A. versicolor (Ebada and Ebrahim, 2020). Aniduquinolone A compound is extracted from ethyl acetate isolated from A. versicolor culture. Additionally, Ibrahim and Asfour (2018) demonstrated that A. versicolor produces aspernolides L, aspernolides M, butyrolactones I, and butyrolactones VI compounds (Fig. 1B–1E), all of which inhibit the growth of methicillin-resistant pathogenic bacteria, including Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli.
Fig. 1
Structures of (A) aniduquinolone A (Ebada and Ebrahim, 2020), (B) aspernolides L, (C) aspernolides M, (D) butyrolactones I, and (E) butyrolactones VI (Ibrahim and Asfour, 2018)
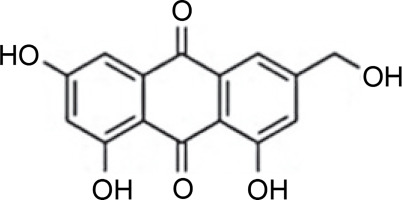
The compound profile of Aspergillus flavus fermentation extract obtained using gas chromatography-mass spectrometry was reported to have various bioactive components. In this case, the main compounds identified were 4-nitrobenzoic acid, 3-chlorophenyl ester (27.23%), and (+)-salsolidine (21.82%) which are nitrobenzoate and alkaloid classes compounds, respectively. These compounds are known to have antimicrobial activities toward Bacillus subtilis, E. coli BL21, Lactococcus lactis, S. aureus, Staphylococcus carnosus, and Staphlococcus simulans (Chowdhury et al., 2018). In addition, Khattak et al. (2021) observed that A. flavus can produce (2E)-3-[(3S, 4R)-8-hydroxy-3, 4-dimethyl-1-oxo-3, 4-dihydro-1H-2-benzo-pyran-7-yl] prop-2-enoic acid compound with the formula of C14H14O5. This compound showed inhibition activity of 58.8% towards MDR strains of S. aureus and 28% towards P. vulgaris.
Endophytic A. flavus GZWMJZ-288, living in symbiosis with Garcinia multiflora, has been reported to produce valuable compounds, including 19-amino-19-dehydroxy 5-epiα-cyclopiazonic acid, 2-hydroxymethyl-5-(3-oxobutan-2-yl)-aminopyran-4(4H)-one, and 4-amino-2 hydroxymethylpyridin-5-ol (Fig. 2). All these compounds exhibit antimicrobial activity against pathogenic bacteria, namely P. aeruginosa ATCC10145, E. coli ATCC11775, S. aureus ATCC6538, S. aureus ATCC25923, and methicillin-resistant S. aureus ATCC43300 (MRSA) (He et al., 2021).
Fig. 2
Structures of (A) 19-amino-19-dehydroxy 5-epi-α-cyclopiazonic acid, (B) 2-hydroxymethyl-5-(3-oxobutan-2-yl)aminopyran-4(4H)-one and (C) 4-amino-2 hydroxymethylpyridin-5-ol (He et al., 2021)
Additionally, A. flavipes has been reported to produce a new indene derivative, methyl 2-(4-hydroxybenzyl)-1,7-dihydroxy-6-(3-methylbut-2-enyl)-1H-indene1-carboxylate, which inhibits the growth of Klebsiella pneumoniae and P. aeruginosa (Akhter et al., 2019). Moreover, bioactive extracts from Aspergillus allahabadii were investigated, revealing the production of the compound allahabadolactones B, exhibiting antibacterial activity against Bacillus cereus (Sadorn et al., 2016). Previous research has documented the antimicrobial, antioxidant, antidiabetic activities, and potential for biocontrol of A. allahabadii by various researchers (Affokpon et al., 2010; Sadorn et al., 2016; Rajamanikyam et al., 2017).
A. fumigatus possesses the ability to produce compounds like pseurotin A, isdethiobis(methylthio)gliotoxin, gliotoxin, spirotryprostatins A, and spirotryprostatins G, all displaying robust antimicrobial activity against pathogenic bacteria E. coli, S. aureus, and C. albicans (Zhang et al., 2018). Additionally, A. fumigatus is known for producing fumiquinazoline-F and fumiquinazoline-D, both exhibiting antibacterial and antifungal activities against B. subtilis, S. aureus, and C. albicans (Shaaban et al., 2013).
A. micronesiensis, an endophytic bacterium, produces cytochalasin A, a compound with antimicrobial activity against S. aureus, methicillin-resistant S. aureus, and C. albicans (Wu et al., 2019). Cytochalasans, a structurally diverse group of secondary metabolites, feature a substituted isoindole scaffold fused with a macrocyclic ring, showing a broad range of biological activities (Skellam, 2017). A. nidulans produces compounds such as 9-octadecenoic acid, methyl ester, methyl stearate, 9,12-octadecadienoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester, and 9,17-octadecadienal, all inhibiting the growth of pathogenic bacteria including S. aureus ATCC 6538,B. cereus ATCC 10,987, B. subtilis ATCC 6633, E. coli ATCC 8739, Salmonella typhimurium ATCC14028, Klebsiella pneumonia ATCC 13,883, P. aeruginosa ATCC 9072, and C. albicans ATCC1023 bacteria (Sharaf et al., 2021). A. niger was further claimed to produce methylsulochrin compound. It is a diphenyl ether derivative that demonstrated antibacterial activity toward pathogenic bacteria, such as S. aureus, Enterobacter cloacae, and Enterobacter aerogenes (Mawabo et al., 2019).
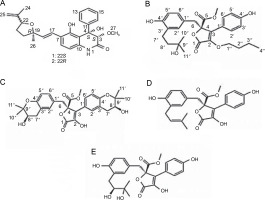
Elkady et al. (2022) successfully extracted endophytic metabolites from A. niger using ethyl acetate, identifying dihydroauroglaucin, isotetrahydroauroglaucin, and cristatumin B compounds (Fig. 3). Antibacterial tests revealed that dihydroauroglaucin exhibited broad antibacterial activity against carbapenem-resistant (CR) K. pneumoniae, methicillin-resistant S. aureus (MRSA), MDR P. aeruginosa, and vancomycin-resistant (VR) Enterococcus faecalis. Isotetrahydroauroglaucin was more effective against Gram-positive bacteria (MRSA and VR E. faecalis) and moderately active against Gram-negative bacteria (CR K. pneumoniae and MDR P. aeruginosa ). Additionally, cristatumin B displayed antibacterial activity across a wide spectrum targeting CR K. pneumoniae and MDR P. aeruginosa (Elkady et al., 2022).
Fig. 3
Structures of (A) dihydroauroglaucin, (B) isotetrahydroauroglaucin, and (C) cristatumin B (Elkady et al., 2022)
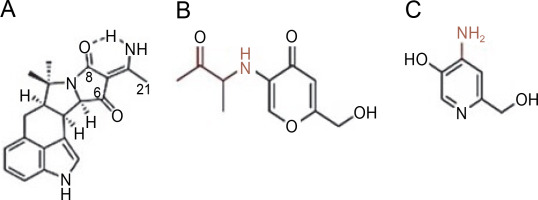
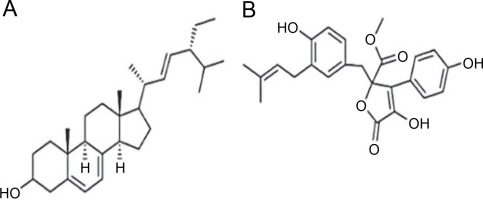
A. terreus has been reported to produce (22E,24R) Stigmasta-5,7,22-trien-3β-ol (Fig. 4A), exhibiting antimicrobial activities against S. aureus and C. neoformans with IC50 values of 28.54 and 4.38 mg/ml, respectively (Elkhayat et al., 2016). (22E,24R)Stigmasta-5,7,22-trien-3b-ol was also effective against methicillin-resistant S. aureus (MRSA) with an IC50 of 0.96 mg/ml. Additionally, A. terreus produces aspernolides F (80), displaying antibacterial activity toward MRSA with an IC50 value of 6.39 mg/ml, and antifungal activity toward C. neoformans with an IC50 value of 5.19 mg/ml (Ibrahim et al., 2015; Elkhayat et al., 2016). da Silva et al. (2017) successfully extracted butyrolactone I from A. terreus using ethyl acetate, revealing its ability to inhibit E. coli (ATCC 25922) at a concentration of 117.6 μM (ATCC 25922).
Fig. 4
Structures of (A) (22E,24R) Stigmasta-5,7,22-trien-3β-ol (Elkhayat et al., 2016), (B) butyrolactone I compounds (da Silva et al., 2017)
It has been reported that A. tubingensis produced rubrofusarin B, 6-isovaleryl-4-methoxy-pyran-2-one, asperpyrones A (102), and campyrone A (103) compounds, inhibiting the growth of E. coli, P. aeruginosa, S. lactis, and S. aureus bacteria (Ma et al., 2015). Previous research by Yang et al. (2019) found that A. tubingensis produced the compound 3-(5-oxo-2,5-dihydrofuran-3-yl) propanoic acid, which exhibited antibacterial activity against S. lactis. Additionally, A. tamarii was reported to produce the compound disulfida cyclo-(Leu-Val-Ile-Cys-Cys) (1), known as malformin E, demonstrating significant antimicrobial activity against B. subtilis, S. aureus, P. aeruginosa, E. coli, P. chrysogenum, C. albicans, and F. solani (Ma et al., 2016).
Penicillium genus
According to reports, endophytic Penicillii demonstrated the ability to colonize ecological niches and protect its host plant against various pressures by displaying a wide range of biological activities, applicable in agricultural, biotechnological, and pharmaceutical contexts (Toghueo and Boyom, 2020). The Penicillium genus, one of the largest fungal groupings, comprises over 200 identified species (Pitt et al., 2000). Metabolites produced by endophytic Penicillii play a crucial role in protecting host plants from pathogenic invasions, particularly through bioactive secondary metabolites with antimicrobial compounds. Yang et al. (2017) reported that Penicillium sp. MZKI P-265 produces helvolic acid, which exhibits inhibitory effects against S. aureus and P. aeruginosa.
The purified fermentation broth of Penicillium sp. HL4-159-41B contains the palitantin compound, showing promising activity in inhibiting the growth of Mycobacterium tuberculosis H37Ra (Li et al., 2015). Another research conducted by Jouda et al. (2016) revealed that penialidin A–C, citromycetin, p-hydroxyphenylglyoxalaldoxime, and brefeldin A have been successfully extracted from Penicillium sp. These compounds demonstrated the ability to inhibit the growth of Vibrio cholerae and Shigella flexneri, with penialidin C exhibiting the best antibacterial activity against Mycobacterium smegmatis. In addition, P. cataractum, another endophytic bacterium, was reported to produce penicimenolidyu A, penicimenolidyu B, and rasfonin compounds, all showing significant activity in inhibiting the growth of S. aureus (Wu et al., 2018).
Four compounds produced by P. ochrochloron (6-(2 R-hydroxy-3 E,5 E-diene-1 -heptyl)-4-hydroxy-3-methyl-2Hpyran-2-one, 6-(2 S-hydroxy-5 E-ene-1 -heptyl)-4-hydroxy-3-methyl-2H-pyran-2-one, 6-(2 S-hydroxy-1 -heptyl)-4-hydroxy-3-methyl-2H-pyran-2-one, and trichodermic acid) were tested for their activity against pathogenic bacteria. The results indicated antibacterial activity against B. subtilis, Micrococcus luteus, S. aureus, Bacillus megaterium, Salmonella enterica, Proteus vulgaris, Salmonella typhi, P. aeruginosa, E. coli, and Enterobacter aerogenes (Zhao et al., 2019). Additionally, another research report claimed that P. ochrochloron produced 4-O-desmethyl-aigialomycin B, penochrochlactones C, and penochrochlactones D, all of which demonstrated the ability to inhibit S. aureus, B. subtilis, E. coli, and P. aeruginosa (Song et al., 2021).
Moreover, P. janthinellum was reported to produce brasiliamide J-a & brasiliamide J-b, peniciolidone, and dehydroaustinol. Brasiliamide J-a & J-b compounds can inhibit the growth of S. aureus, B. subtilis, P. aeruginosa, K. pneumonia, and E. coli, while peniciolidone and dehydroaustinol were found to inhibit S. aureus, B. subtilis, and E. coli (Xie et al., 2018). P. citrinum was able to produce citrinin and emodin; citrinin exhibited antifungal activity against the plant pathogenic fungus Alternaria citri, while emodin had antifungal activity against the pathogenic fungus Bipolaris maydis (Luo et al., 2019).
Penicillium setosum was reported to synthesize kaempferol, patulin, leucodelphinidin, quercetin, and dihydroquercetin compounds, all exhibiting antibacterial activity against E. coli and S. aureus (George et al., 2019). Penicillium vulpinum was found to contain (−)-3-carboxypropyl-7-hydroxyphthalide and (−)-3-carboxypropyl-7-hydroxyphthalide methyl ester compounds. Specifically, (−)-3-carboxypropyl-7-hydroxyphthalide inhibited the growth of Bacillus subtilis, Shigella dysenteriae, and Enterobacter aerogenes with an MIC value of 12.5–25 μg/ml Meanwhile, (−)-3-carboxypropyl-7-hydroxyphthalide methyl ester displayed antibacterial activity against E. aerogenes with a MIC value of 12.5 μg/ml (Qin et al., 2019). Qin et al. (2020) also reported the production of 10-demethylated andrastone A, 15-deacetylcitreohybridone E, citreohybridonol, andrastins A, and andrastins B by P. vulpinum. All these compounds displayed inhibitory activity against B. megaterium. Notably, citreohybridonol exhibited strong inhibitory activity against B. paratyphosus and moderate inhibitory activity against E. coli and S. aureus.
Penicillium vinaceum produced (−)-(1R,4R)-1,4-(2,3)indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione, an alkaloid quinazoline compound inhibiting the growth of C. albicans ATCC 76615 and C. neoformans ATCC 32609 (Zheng et al., 2012). Penicillium brefeldianum produced p-hydroxybenzaldehyde, a compound also found in Syzygium zeylanicum root bark that exhibited inhibitory activity against S. typhi, E. coli, and B. subtilis (Syarifah et al., 2021). Penicillium restrictum is known to produce polyhydroxyanthraquinone (Fig. 5), demonstrating inhibitory activity against MRSA (Graf et al., 2020). Furthermore, Penicillium sumatrense secreted citridone E and (–)-dehydrocurvularin compounds with antibacterial activity against S. aureus, P. aeruginosa, Clostridium perfringens, and E. coli (Xu et al., 2019).
Out of a total of 95 species of both Aspergillii and Penicillii known for their antimicrobial and multidrugresistant pathogen inhibitory activity, 55.8% were identified as Aspergillus and 44.2% as Penicillium (Fig. 6A). Further analysis of several articles revealed that endophytic fungi of Aspergillus and Penicillium inhibit multidrug resistant pathogens by only 8.4%, while the remaining 91.6% target nonMDR microorganisms (Fig. 6B).
Fig. 6
A) proportion of studies (N = 50) reporting Aspergillii and Penicillii from medicinal plants, B) proportion of studies (N = 50) reporting Aspergillii together with Penicillii targeting antibiotics-resistant and nonresistant microbes
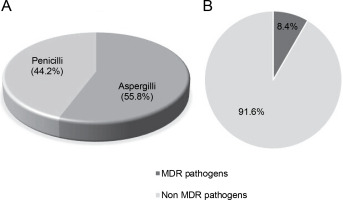
Analysis of multiple articles indicates that certain species of Aspergillus and Penicillium possess antimicrobial activity against MDR pathogens. Consequently, it is imperative to continue exploring endophytic fungi to identify and assess their potential to produce bioactive compounds capable of eradicating pathogenic microorganisms, including MDR pathogens, in nature.
Host plants
Endophytic fungi establish a mutually beneficial relationship with their host plants. They receive food and protection from the host plant, while the plant benefits from increased resistance to both abiotic and biotic stresses (Saikkonen et al., 2015; Mamangkey et al., 2022b).
Due to this symbiotic association, endophytic bacteria residing alongside plant tissues generate bioactive compounds akin to those synthesized by the plants themselves (Mamangkey et al., 2019; Munir et al., 2019). Medicinal plants are widely valued for their potential in disease prevention, owing to their renowned abundance of natural constituents (Yirga et al., 2011; Pan et al., 2013). Ultimately, these medicinal plants serve as natural habitats for the colonization of endophytic fungi, which, in turn, produce multifunctional compounds.
Endophytic bacteria and fungi are microorganisms that can be isolated from various parts of medicinal plants, including roots, stems, leaves, fruits, seeds, rhizomes, and tree barks (Mamangkey et al., 2019; Mamangkey et al., 2020; Mamangkey et al., 2022a). While both categories of endophytes, namely bacteria and fungi, are capable of producing bioactive compounds, fungi are more commonly isolated and have exhibited a higher propensity for generating a broader array of secondary metabolites. This remarkable diversity significantly enhanced the likelihood of uncovering novel antibacterial agents (Radić and Strukelj, 2012). Endophytic fungi that produce bioactive chemicals have a wide range of inherent qualities, including anti-inflammatory, antimicrobial, anticancer, antidiabetic, and antibiotic (Ruma et al., 2013; Mamangkey et al., 2022a, 2022b, 2023). The tabulation of information and reports on several documented Aspergillii and Penicillii originating from medicinal plants can be seen in Table 1.
Table 1
Symbiotic Aspergilli and Penicilli species in medicinal plants
| Endophyte fungi | Host species | Reference |
|---|---|---|
| Aspergillus sp. TJ23 | Hypericum perforatum | Qiao et al., 2018 |
| Aspergillus sp. DTE1 | Medinilla speciosa | Amelia et al., 2021 |
| Aspergillus sp. TR_L1 | Tabebuia rosea | Elkady et al., 2022 |
| A. ochraceus | Bauhinia forficate | Bezerra et al., 2015 |
| A. neobridgeri | Pistacia lentiscus | Sadrati et al., 2020 |
| A. niger (OL519514) | Opuntia ficus-indica | Elkady et al., 2022 |
| A. versicolor | Eichhornia crassipes Pulicaria crispa | Ebada and Ebrahim, 2020; Ibrahim and Asfour (2018) |
| A. flavipes | Suaeda glauca | Akhter et al., 2019 |
| A. flavus | Garcinia multiflora | He et al., 2021 |
| A. allahabadii | Cinnamomum subavenium | Sadorn et al., 2016 |
| A. flavus | Corchorus olitorius Mentha piperetta | Chowdhury et al., 2018; Khattak et al. 2021 |
| A. fumigatus | Edgeworthia chrysantha Ipomoea batatas | Zhang et al., 2018; Shaaban et al., 2013 |
| A. micronesiensis | Phyllanthus glaucus | Wu et al., 2019 |
| A. nidulans | Ocimum basilicum | Sharaf et al., 2021 |
| A. tamarii | Ficus carica | Ma et al., 2016 |
| A. niger | Acanthus montanus Opuntia ficus-indica | Mawabo et al., 2019; Elkady et al., 2022 |
| A. terreus | Carthamus lanatus Hyptis suaveolens | Ibrahim et al., 2015; Elkhayat et al., 2016; da Silva et al. 2017 |
| A. tubingensis | Lycium ruthenicum Decaisnea insignis | Ma et al., 2015; Yang et al., 2019 |
| A. tamarii | Ficus carica | Ma et al., 2016 |
| Penicillium sp. | Aralia nudicaulis Garcinia nobilis Curcuma longa Melia azedarach L. Murraya paniculata (L.) Jack Schinus terebinthifolius Pinellia ternate | Li et al., 2015; Jouda et al., 2016; Singh et al., 2014; Pastre et al., 2007; Tonial et al., 2016; Yang et al. 2017 |
| P. ochrochloron | Taxus media Kadsura angustifolia | Zhao et al., 2019; Song et al., 2021 |
| P. janthinellum | Panax notoginseng | Xie et al., 2018 |
| P. setosum | Withania somnifera | George et al., 2019 |
| P. vulpinum | Sophorae tonkinensis | Qin et al., 2019; Qin et al., 2020 |
| P. vinaceum | Crocus sativus | Zheng et al., 2012 |
| P. brefeldianum | Syzygium zeylanicum | Syarifah et al., 2021 |
| P. restrictum | Silybum marianum (L) Gaertn. | Graf et al., 2020 |
| P. sumatrense | Garcinia multiflora | Xu et al., 2019 |
| P. citrinum | Stephania kwangsiensis | Luo et al., 2019 |
| P. cataractum | Ginkgo biloba | Wu et al., 2018 |
| P. ochrochloronthe | Taxus media | Zhao et al., 2019 |
| P. roqueforti | Solanum surattense | Ikram et al., 2019 |
| P. minioluteum | Orthosiphon stamineus Benth | Tong et al., 2014; Rozman et al., 2017 |
| P. amestolkiae | Orthosiphon stamineus Benth | |
| P. purpurogenum | Swietenia macrophylla | Yenn et al., 2017 |
| P. setosum | Withania somnifera | George et al., 2019 |
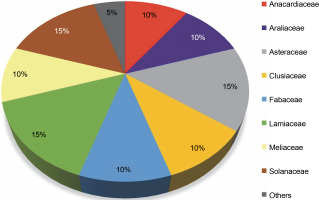
This review highlights 44 medicinal plant species that act as natural hosts for endophytic fungi, particularly within the Aspergillus and Penicillium genera. These plants span across 20 different families, as illustrated in Figure 7. Among these medicinal plant species, 15% prominent families have surfaced: Asteraceae, Lamiaceae, and Solanaceae, with each family comprising three species. Additionally, families such as Anacardiaceae, Araliaceae, Clusiaceae, Fabaceae, and Meliaceae each have 10% species, while 25 other medicinal plant families have 5% species. The prevalence of Aspergillus and Penicillium endophytes in these 44 medicinal plant species, especially within the Asteraceae, Lamiaceae, and Solanaceae families, suggests that these plants contain a diverse range of bioactive compounds effective against pathogenic fungi and bacteria, including MDR pathogens. These plants likely possess a well-organized system of chemical defense mechanisms, enabling them to protect themselves from diseases and environmental stress. Simultaneously, they offer a potential source of novel drugs for human use.
Fig. 7
Proportion of plant species within families (N = 20) harboring endophytic Aspergillii and Penicillii
Research has indicated the presence of endophytic microbiota in various plant groups, from thallophytes to spermatophytes, and across diverse habitats from hydrophytes to xerophytes (Verma et al., 2017a, 2017b, 2017c). Caruso et al. (2020) highlighted the presence of various medicinal plant species within the Asteraceae family. Numerous studies have documented the pharmacological activity and chemical constituents of Asteraceae plants, revealing the presence of polyphenols, sesquiterpenes, organic acids, and fatty acids. These compounds have been associated with the effective treatment of various health conditions, including cardiovascular diseases, cancer, microbial and viral infections, inflammation, and other ailments (Morales et al., 2014).
The Lamiaceae (Labiatae) family, known as “lumbase nilcols” in Asian countries, stands out as a rich source of essential oils extensively utilized in the food, pharmaceutical, and cosmetic industries. These plants are characterized by secondary metabolites with antibacterial, antifungal, antioxidant, anti-inflammatory, and antiviral properties (Mesquita et al., 2019; Mamangkey et al., 2023). Typically, endophytic bacteria produce these secondary metabolites, utilizing plant cells as a source of nutrition. The Solanaceae family, comprising over 2000 species across 90 genera, has substantial commercial value in both the food and medicinal industries, as reported by Petkova et al. (2021). Previous research on other Solanaceae members has revealed systematic colonization by endophytic fungi within these plants (Tefera and Vidal, 2009; Jaber and Enkerli, 2016; Jaber and Araj, 2018). The ability of endophytic fungi to colonize Solanaceae plants suggests that specific growth requirements are adequately met within this family.
Conclusion
Aspergillus and Penicillium endophytic fungi are recognized for their antimicrobial properties, particularly against multidrug-resistant pathogens. Among the medicinal plants under study, those belonging to the Asteraceae, Lamiaceae, and Solanaceae families have garnered significant attention due to their association with these endophytes. The rich diversity of bioactive compounds systematically synthesized within these plants may elucidate the potential of endophytic Aspergillii and Penicillii fungi in inhibiting and eradicating pathogenic microbes. As a result, these fungi have the potential to serve as sources for new antibiotics, offering potential solutions for controlling infections caused by MDR pathogens. The development of novel antibiotics from endophytic fungi associated with medicinal plants is an exciting prospect for the pharmaceutical industry.