Introduction
In many African households, cereal grains account for about 77% of total food energy intake, according to the report by Onipede et al. (2021). Ogi is a well-documented indigenous fermented food made from cereal grains such as maize, millet, and sorghum, and is widely consumed across West Africa, particularly in Nigeria (Ijarotimi et al. 2022). The production of ogi involves dehulling, sorting, washing, and wet-milling. The filtrate is then allowed to ferment spontaneously for about 48–72 h (Omemu et al. 2018).
Lactic acid bacteria (LAB) and yeasts are the predominant microorganisms involved in the fermentation of cereal grains. They play key roles in acidifying the raw materials and producing organic acids such as lactic, acetic, citric, and succinic acids (Onipede et al. 2021; Ozabor et al. 2022). These organisms have also been documented in the literature to possess healthpromoting properties that make them suitable for functional food production. Some of these properties include α-amylase and α-glucosidase inhibitory activity, and the production of gamma-aminobutyric acid (GABA), as reported by Gabaza et al. (2019), Laranjo et al. (2019), Banwo et al. (2021), and Liu et al. (2021).
Hydroxylmethylglutaryl-coenzyme A reductase (HMGCoA reductase) is a key enzyme involved in cholesterol biosynthesis. Inhibiting the activity of HMG-CoA reductase is essential for reducing cholesterol synthesis by modulating low-density lipoprotein receptors (Marahatha et al. 2021). Additionally, the angiotensin-converting enzyme (ACE) regulates blood pressure by converting angiotensin I (Ang I) into angiotensin II (Ang II), a vasoconstrictor that narrows blood vessels and increases blood pressure, eventually leading to hypertension (Wang et al. 2014; Filippou et al. 2020). This occurs through the interaction of Ang II with the type I receptor (Xia et al. 2020). Together, inhibition of HMG-CoA reductase and ACE is crucial for lowering blood cholesterol and blood pressure, respectively.
“Statins” such as simvastatin and “prils” such as captopril are chemically synthesized drugs developed in the mid-1970s for treating hyperlipidemia and hypertension, respectively (Rinto et al. 2017; Chen et al. 2021). However, several studies have reported that long-term use of these drugs can cause adverse side effects, potentially leading to organ damage (Ramkumar et al. 2016; Li et al. 2020; Huang et al. 2021). This has necessitated the exploration of alternative natural sources of HMGCoA reductase and ACE inhibitors, among which LAB and yeasts have shown promise.
Some fermented food products – such as bekasam, and green and black teas fermented with LAB and yeasts – have been previously reported to inhibit ACE and HMG-CoA reductase (Gamboa-Gomez et al. 2016; Rinto et al. 2017). Starter culture-fermented dairy products such as yogurt and milk have also been documented by Xia et al. (2020) to inhibit ACE activity. According to Banwo et al. (2022), the inhibitory effects observed in LAB and yeasts may be due to the production of bioactive peptides released into the fermenting matrix during fermentation. Microbial genera reported in previous studies to contribute to HMG-CoA reductase and ACE inhibition include Lactococcus, Lactobacillus, Candida, Xanthomonas, Streptomyces, Bacillus, and Actinomadura (Rinto et al. 2017; Li et al. 2020).
Although several studies in Nigeria have reported HMG-CoA reductase and ACE inhibitory activities by botanicals, to the best of our knowledge, this is the first report documenting such inhibitory activities by LAB and yeasts isolated in Nigeria as alternative natural inhibitors without known side effects.
Therefore, this study aimed to investigate the in vitro inhibition of HMG-CoA reductase and ACE by LAB and yeasts isolated from spontaneously fermented sorghum gruels, with potential application as starter cultures in the fermentation of functional foods or nutraceuticals.
Materials and methods
Collection and fermentation of sorghum grains
Spontaneously fermented sorghum ogi was prepared according to the method described by Ozabor et al. (2022). White and red sorghum grains were purchased from Olu-Ode Market in Osogbo, Osun State, Nigeria, and identified in the Department of Plant Biology, Osun State University, Osogbo. The grains were packaged in low-density polyethylene bags. Foreign materials such as dirt, broken grains, and stones were removed manually, and the grains were thoroughly washed with potable water. Five hundred grams (500 g) of each grain sample were steeped in distilled water for 2 days (48 h), wet-milled, sieved, and allowed to ferment spontaneously for 72 h, following the procedure described by Ozabor et al. (2022).
Serial dilution
The fermented sorghum was serially diluted tenfold. Ten grams (10 g) of each fermentate were weighed and transferred into sterile test tubes containing 90 ml of sterile distilled water to form the stock solution. One (1) ml of the stock was then dispensed into nine test tubes, each containing 9 ml of sterile distilled water, and arranged in test tube racks. Dilution factors of 105 and 107 were spread-plated onto sterile Petri dishes containing MRS agar and YEA for the enumeration of LAB and yeasts, respectively (Ojokoh et al. 2014).
Isolation of LAB and yeasts
Lactic acid bacteria and yeasts were cultured on de Man, Rogosa, and Sharpe (MRS) agar and yeast extract agar (YEA), respectively (HiMedia Laboratories, Kennett Square, USA). LAB cultures were incubated anaerobically at 37°C for 18–24 h while yeast cultures on YEA were incubated aerobically at 30°C for 18–24 h.
Molecular identification of LAB and yeasts
The isolated LAB and yeasts were identified molecularly using polymerase chain reaction (PCR) and Sanger sequencing techniques. Genomic DNA was extracted following the manufacturer’s instructions using the Quick-DNATM Miniprep Plus Kit (catalog nos. D4068 and D4069, Zymo Research). The Lb F/R primers (5′-GAGTTTGATCCTGGCTCAG-3′/5′-AGAAAG-GAGGTGATCCAGCC-3′) were used for LAB identification, while ITS4 and ITS5 primers (5′-TC-CTCCG-CTTATTGATATGC-3′/5′GGAAGTAAAAGTCGTAACAA-GG-3′) were used for yeast identification.
The PCR protocol for LAB was: 95°C for 3 min (initial denaturation), followed by 94°C for 30 s (denaturation), 50°C for 30 s (annealing), 72°C for 90 s (extension), and a final elongation at 72°C for 5 min. The PCR protocol for yeast isolates was: 95°C for 10 min (initial denaturation), followed by 94°C for 30 s (denaturation), 55°C for 30 s (annealing), 72°C for 1 min (extension), and final elongation at 72°C for 7 min (Angelov et al. 2017; Tilahun et al. 2018; Banwo et al. 2021).
The evolutionary distances for the phylogenetic trees were computed using the Maximum Composite Likelihood method.
The nucleotide sequences of the identified LAB and yeast isolates were submitted to the National Center for Biotechnology Information (NCBI) database, and the assigned accession numbers are as follows: Candida tropicalis RSY43 (PP110435); Cryptococcus sp. RSY48 (PP110438); Cryptococcus albidus RSY51 (PP110440); Naganishia albida WSY40 (PP110441); Lactobacillus pentosus WSL5 (PP115584); L. plantarum RSL1 (PP115585); and L. delbrueckii RSL11 (PP115587).
Screening for hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitory activities by LAB and yeast strains
The HMG-CoA reductase inhibitory activity of the LAB and yeast isolates was determined based on spectrophotometric measurements. Twenty-four-hour-old cultures of LAB and yeasts were aseptically transferred into Eppendorf tubes, lyophilized, and weighed. Each sample was mixed with a reaction mixture containing nicotinamide adenine dinucleotide phosphate (NADPH, 400 µM), HMG-CoA substrate (400 µM), and 100 mM potassium phosphate buffer (pH 7.4) supplemented with potassium chloride (120 mM), ethylenediaminetetraacetic acid (EDTA, 1 mM), and dithiothreitol (DTT, 5 mM). This was followed by the addition of HMG-CoA reductase (2 µl). The reaction mixture was incubated at 37°C, and absorbance was measured at 340 nm after 10 min. Simvastatin (Sigma-Aldrich Co.) was used as a positive control and distilled water as a negative control (Dewanti et al. 2017; Jaipal et al. 2022).
The percentage of HMG-CoA reductase inhibition was calculated using the following formula:
Screening for angiotensin-converting enzyme (ACE) inhibitory activities by LAB and yeast strains
The ACE inhibition assay was conducted using a spectrophotometric method. Twenty-four-hour-old cultures of LAB and yeasts were aseptically transferred into Eppendorf tubes, lyophilized, and weighed. Stock solutions were prepared and serially diluted in 0.1 M potassium phosphate buffer containing 0.2 M sodium chloride (NaCl) to obtain different concentrations.
Fifty microliters (50 µl) of each sample and 50 µl of ACE (0.05 mU/µl) were mixed and preincubated at 37 °C for 10 min. Then, 150 µl of 6.5 mM hippuryl-histidiyl-leucine (HHL) solution was added, and the reaction mixture was incubated at 37°C for 60 min. The reaction was terminated by adding 250 µl of 1 M HCl. The reaction product, hippuric acid (HA), was extracted by adding 1.5 ml of ethyl acetate. The mixture was centrifuged at 3000 rpm for 15 min, and 1 ml of the supernatant was transferred to a 5 ml test tube for ethyl acetate removal. The tubes were placed in a water bath at 80°C until complete evaporation. The resulting residues were re-dissolved in 1 ml of deionized water before UV-VIS measurement at 228 nm. Absorbance was measured in duplicate using deionized water as the blank (Geogalaki et al. 2017; Ahmed et al. 2018).
Statistical analysis
Values are presented as means ± standard error from triplicate measurements at four independent concentrations. An asterisk (*) indicates statistical significance compared to the control at p < 0.05. Differences in means were analyzed using one-way ANOVA (analysis of variance) at α = 0.05.
Results
The ACE and HMG-CoA reductase inhibitory activities of LAB
The percentage ACE and HMG-CoA reductase inhibitory activities of the LAB isolates used in this study are shown in Figure 1. The isolates include Lactobacillus plantarum RSL1 (Figure 1A), L. delbrueckii RSL11 (Figure 1B), L. pentosus WSL1 (Figure 1C), and L. pentosus WSL5 (Figure 1D).
Figure 1
A) Angiotensin converting enzyme (ACE) and 3-hydroxyl-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitory activity produced by L. plantarum RSL1. *Significant when compared with control at p < 0.05. B) ACE and HMG-CoA reductase inhibitory activity produced by L. delbrueckii RSL11. *Significant when compared with control at p < 0.05. C) ACE and HMG-CoA reductase inhibitory activity produced by L. pentosus WSL1. *Significant when compared with control at p < 0.05. D) ACE and HMG-CoA reductase inhibitory activity produced by L. pentosus WSL5. *Significant when compared with control at p < 0.05
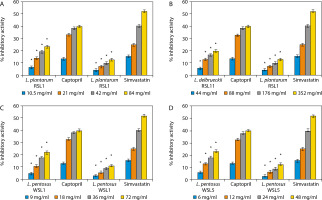
It was observed that increasing the concentrations of LAB correspondingly increased both ACE and HMG-CoA reductase inhibitory activities. At concentrations of 6, 12, 24, and 48 mg/ml, L. pentosus WSL5 exhibited the highest ACE inhibitory activities of 6.38, 13.17, 18.13, and 23.47%, respectively, along with HMG-CoA reductase inhibitory activities of 2.21, 6.42, 9.17, and 12.84%, respectively.
Conversely, L. delbrueckii RSL11, at concentrations of 44, 88, 176, and 352 mg/ml, showed the lowest ACE inhibitory activities of 5.72, 13.17, 16.60, and 20.04%, respectively, and HMG-CoA reductase inhibitory activities of 4.59, 7.33, 10.10, and 13.30%, respectively.
The ACE and HMG-CoA reductase inhibitory activities of yeasts
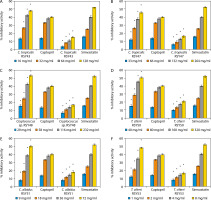
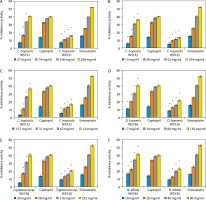
The percentage ACE and HMG-CoA reductase inhibitory activities of the selected yeast strains are presented in Figure 2. The strains include Candida tropicalis RSY43 (Figure 2A), C. tropicalis RSY47 (Figure 2B), Cryptococcus sp. RSY48 (Figure 2C), C. albidus RSY51 (Figure 2D), Trichomonascus ciferri RSY53 (Figure 2E), and C. tropicalis WSY31 (Figure 2F).
Figure 2
A) Angiotensin converting enzyme (ACE) and 3-hydroxyl-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitory activities of C. tropicalis RSY43. *Significant when compared with control at p < 0.05. B) ACE and HMG-CoA reductase inhibitory activities of C. tropicalis RSY47. *Significant when compared with control at p < 0.05. C) ACE and HMG-CoA reductase inhibitory activities of Cryptococcus sp. RSY48. *Significant when compared with control at p < 0.05. D) ACE and HMG-CoA reductase inhibitory activities of T. ciferri RSY50. *Significant when compared with control at p < 0.05. E) ACE and HMG-CoA reductase inhibitory activities of C. albidus RSY51. *Significant when compared with control at p < 0.05. F) ACE and HMG-CoA reductase inhibitory activities of T. ciferri RSY53. *Significant when compared with control at p < 0.05
An increase in yeast concentration resulted in a corresponding increase in %ACE and HMG-CoA reductase inhibitory activities. At concentrations of 1, 2, 4, and 8 mg/ml, T. ciferri RSY53 exhibited the highest ACE inhibitory activities of 11.83, 20.91, 34.73, and 48.28%, respectively, alongside HMG-CoA reductase inhibitory activities of 7.71, 11.47, 14.68, and 16.97%, respectively. Conversely, C. tropicalis WSY31 showed the lowest ACE inhibitory activities of 6.87, 16.79, 34.16, and 40.83%, and HMG-CoA reductase inhibitory activities of 6.8, 10.55, 13.30, and 16.06% at concentrations of 37, 74, 148, and 296 mg/ml, respectively.
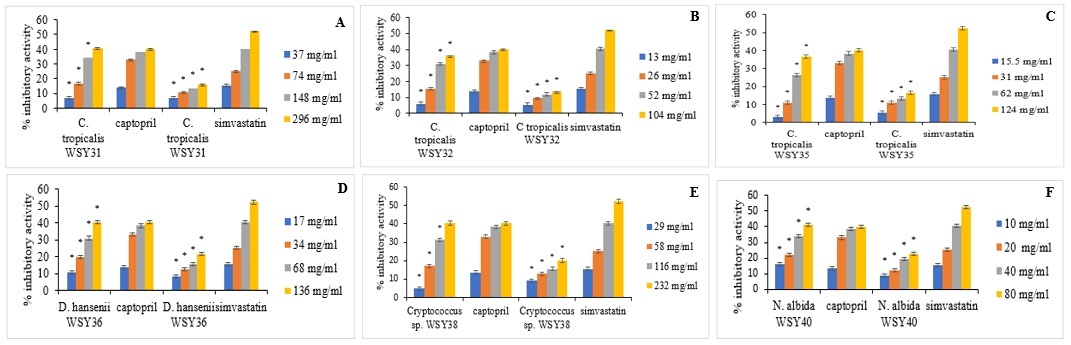
Notably, the yeast isolates demonstrated higher ACE inhibitory activity than the standard reference drug (captopril), and their HMG-CoA reductase inhibitory activity also increased in a concentration-dependent manner (Figure 3). The yeast strains presented in Figure 3 include C. tropicalis WSY31 (Figure 3A), C. tropicalis WSY32 (Figure 3B), C. tropicalis WSY35 (Figure 3C), Debaryomyces hansenii WSY36 (Figure 3D), Cryptococcus sp. WSY38 (Figure 3E), and Naganishia albida WSY40 (Figure 3F).
Figure 3
A) Angiotensin converting enzyme (ACE) and 3-hydroxyl-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitory activities of C. tropicalis WSY31. *Significant when compared with control at p < 0.05.B) ACE and HMG-CoA reductase inhibitory activities of C. tropicalis WSY32. *Significant when compared with control at p < 0.05.C) ACE and HMGCoA reductase inhibitory activities of C. tropicalis WSY35. *Significant when compared with control at p < 0.05.D) ACE and HMG-CoA reductase inhibitory activities of D. hansenii WSY36. *Significant when compared with control at p < 0.05.E) ACE and HMG-CoA reductase inhibitory activities of Cryptococcus sp. WSY38. *Significant when compared with control at p < 0.05. F) ACE and HMG-CoA reductase inhibitory activities of N. albida WSY40. *Significant when compared with control at p < 0.05
Half-maximal inhibitory concentration (IC50) connotes the concentration at which LAB, yeasts, or standard controls (simvastatin and captopril) inhibit HMG-CoA reductase or ACE activity by 50%. Lower IC50 values indicate lower systemic toxicities which implies that when the IC50 value (s) is low, inhibitory activity by the microbial isolates can be achieved at a very minimum concentration. Thus, establishing the effectiveness of the LAB and yeasts.
Among the LAB, L. pentosus WSL5 recorded the lowest HMG-CoA reductase IC50 value of 219.72 µg/ml and ACE IC50 value of 116.22 µg/ml, while L. delbrueckii RSL11 showed the highest IC50 values – 1700.47 µg/ml for HMG-CoA reductase and 1057.49 µg/ml for ACE.
Among the yeast organisms, the lowest HMG-CoA reductase inhibitory IC50 value of 29.55 µg/ml with ACE inhibitory IC50 value of 7.03 µg/ml was obtained from T. ciferri RSY53 while C. tropicalis WSY31 was recorded to have the highest HMG-CoA reductase inhibitory IC50 value of 1296.77 µg/ml with an ACE inhibitory IC50 value of 331.93 µg/ml as documented in Table 1.
Table 1
Half-maximal inhibitory concentration (IC50) values for 3-hydroxyl-3-methylglutaryl-coenzyme A (HMG-CoA) reduc-tase and angiotensin converting enzyme (ACE) inhibitions produced by the selected lactic acid bacteria (LAB) and yeast strains
Discussion
The search for natural sources of bioactive peptides with potential therapeutic applications has gained significant attention in recent years. Among the diverse microbial world, LAB and yeasts have emerged as promising candidates, exhibiting bioactivities that could contribute to the development of novel functional foods and biotherapeutic agents. ACE plays an important role in blood pressure regulation through its involvement in the renin-angiotensin-aldosterone system, while HMGCoA reductase is a key enzyme in cholesterol biosynthesis (Xia et al. 2020; Jaipal et al. 2022). Therefore, the inhibitions of HMG-CoA reductase and ACE is a key approach targeted towards the treatment of hyperlipidemia and hypertension. Although there are clinical drugs for the inhibitions of HMG-CoA reductase and ACE, studies have shown that they produce deleterious effects that can lead to organ damage (Daliri et al. 2018; Galli et al. 2019), hence, the need for natural, novel and nontoxic sources of HMG-CoA reductase and ACE inhibitors.
Therefore, this study contributes valuable insight into the HMG-CoA reductase and ACE inhibitory activities exhibited by LAB and yeasts isolated from ogi, a spontaneously fermented sorghum gruel commonly consumed in Nigeria. The findings support the potential application of these isolates as starter cultures in the production of natural, safe, and novel functional foods and/or nutraceuticals specifically targeted at managing cholesterol and blood pressure through enzyme inhibition.
In this study, it was observed that as the concentration of LAB increased, both ACE and HMG-CoA reductase inhibitory activities also increased. This observation aligns with the earlier findings of Li et al. (2020). ACE inhibitory activity has previously been recorded in L. plantarum, Bifidobacterium animalis, and Streptococcus thermophilus used as starter cultures for milk fermentation (Dewanti et al. 2017). Daliri et al. (2018) also reported ACE inhibitory activities in Pediococcus acidilactici SDL1414, L. plantarum JDFM44, Enterococcus faecium SC54, P. acidilactici DM9, Lactobacillus brevis SDL1411, Pediococcus pentosaceus SDL1409, and Lacticaseibacillus rhamnosus JDFM6 isolated from whey proteins and used as starter cultures for nutraceutical production – results consistent with the findings of this present study.
The HMG-CoA reductase inhibitory activity of LAB has also been documented by Tsai et al. (2014), who reported LAB strains with cholesterol-lowering properties, supporting the results of this work. ACE-inhibiting species of Lactobacillus sp., Enterococcus sp., Streptococcus sp., and Lactococcus sp. have also been isolated from traditional Greek dairy products by Georgalaki et al. (2017). In addition, Biswas et al. (2014), Rodriguez et al. (2019), and Xia et al. (2020) reported ACE inhibition from aqueous tomato extracts, LAB from blue corn hydrolysates, and milk products fermented with L. plantarum QS670, respectively.
Moreover, the study by Dewanti et al. (2017) reported that L. acidophilus isolated from fermented bekasam in Indonesia inhibited HMG-CoA reductase, which aligns with the present findings. Fan et al. (2022) further investigated ACE and HMG-CoA reductase inhibitory activities in microbial isolates, substantiating the trends observed in this study.
ACE inhibition by LAB has also been reported by Chen et al. (2021) and Glazunova et al. (2022) from plant extracts, L. delbrueckii LB100, and L. lactis AM1 used as starter cultures for dairy fermentation. HMG-CoA reductase inhibition in Gryllus bimaculatus fermented by Bacillus sp. and Lactobacillus strains was reported by Jang and Kim (2021). Similarly, Yadav et al. (2019) documented HMG-CoA reductase inhibition by L. rhamnosus MTCC:5957 and MTCC:5897 employed as starter cultures for dairy fermentation targeted at ameliorating hyperlipidemia.
The ACE and HMG-CoA reductase inhibitory activities demonstrated by the yeast strains were also observed to increase in a concentration-dependent manner. Previous studies by Lachenmeier et al. (2012), Ni et al. (2012), Burke (2015), and Jaipal et al. (2022) have reported HMG-CoA reductase inhibitory activity from Monascus sp., M. purpureus, and Prosopis cineraria extract. Red yeast rice, fermented by Monascus species, has been widely consumed as a food supplement for managing hyperlipidemia. These findings are consistent with the results obtained in the present study.
Similarly, Pavon et al. (2013) and Georgalaki et al. (2017) previously reported HMG-CoA reductase inhibitory activity from fungi, demonstrating their potential as biotherapeutic agents in animal models. Furthermore, Hipol et al. (2020) and Rahmi et al. (2022) documented HMG-CoA reductase activity in Cryptococcus sp., Trichosporon sp., Colletotrichum sp., and endophytic fungi isolated from lemongrass.
In addition, studies by Mirzaei et al. (2016) and Li et al. (2022) reported antioxidant and ACE inhibitory activities from Saccharomyces cerevisiae and the mushroom Stropharia rugosoannulata, respectively – supporting the current findings. Ansor et al. (2013) and Rai et al. (2015) also documented ACE-inhibitory peptides from the mycelia of Ganoderma lucidum and fermented dairy products, both targeted at reducing oxidative stress and preventing cardiovascular diseases such as hypertension and hyperlipidemia. Other sources of ACE and HMG-CoA reductase inhibition reported in the literature include algae (Ko et al. 2023). Although such inhibitory effects have predominantly been reported from botanicals (Chakraborty and Roy 2021; Huang et al. 2021), the use of food-grade microorganisms as novel inhibitors is an emerging area of interest.
The half-maximal inhibitory concentration (IC50) represents the concentration at which LAB and yeast starter cultures inhibit ACE and HMG-CoA reductase by 50%. Lower IC50 values indicate reduced systemic toxicity, as effective enzyme inhibition is achieved at lower concentrations. According to Daliri et al. (2018), IC50 values (%) of 19.78, 65.53, 70.50, 96.70, 1280.00, 2070.00, and 2130.00 were obtained from P. acidilactici SDL1414, L. plantarum JDFM44, Enterococcus faecalis SC54, P. acidilactici DM9, L. brevis SDL1411, P. pentosaceus SDL1409, and L. rhamnosus JDFM6, respectively, all isolated from whey proteins – results that align with this study.
Xia et al. (2020) also reported similar IC50 values from L. plantarum isolated from whey proteins in milk. Comparable results were observed in the study by Rinto et al. (2017), which documented IC50 values from Lactobacillus species isolated from bekasam. In support, Glazunova et al. (2022) reported similar ACE inhibitory IC50 values from milk fermented with co-cultures of L. delbrueckii, Lacticaseibacillus paracasei, and Streptococcus thermophilus.
Therefore, due to the lower IC50 values obtained from the yeasts and LAB isolates documented in this study when compared to the controls (captopril and simvastatin), especially T. ciferri RSY53 and L. pentosus WSL5, it can be inferred that these microbial strains are better and novel inhibitors of HMG-CoA reductase and ACE than the known clinical drugs (simvastatin and captopril) since high enzymes inhibition can be achieved at low concentrations of the LAB and yeast functional starter cultures.
Conclusions
Taken together, the collective evidence from this study underscores the potential of the isolated LAB and yeast strains in inhibiting ACE and HMG-CoA reductase activities. Among the isolates, the LAB strain Lactobacillus pentosus WSL5 and the yeast strain Trichomonascus ciferri RSY53 exhibited the highest enzyme inhibitory activities, accompanied by the lowest IC50 values. These findings suggest that L. pentosus WSL5 and T. ciferri RSY53 could serve as promising starter cultures for fermentation processes aimed at producing functional foods designed to ameliorate hyperlipidemia and hypertension through the inhibition of HMG-CoA reductase and ACE.
Furthermore, future research can be directed towards developing functional food formulations using L. pentosus WSL5 and T. ciferri RSY53 as starter cultures, conducting in vivo assays to validate their enzyme inhibitory effects, and identifying and characterizing the genes responsible for these activities in the selected LAB and yeast strains.