Introduction
Industrialization and other human activities that generate toxic aqueous effluents containing synthetic dyes and other organic and inorganic pollutants are accumulating a huge pressure on aquatic environments (Fomina and Gadd, 2014). The increasing demand for textile dyes and other dyeing-related products has led to the discharge of untreated effluents into the environment (Agrawal and Verma, 2019a).
Human beings produce more than 1 million tons of artificial dyes annually, and about 280 000 tons of them are discharged as effluents from textile industries. The structural diversity of artificial dyes is attributable to the presence of different chromophore groups such as azo, anthraquinone (AQ), and triphenylmethane (Mishra and Maiti, 2018). AQ dyes are the second most important class of textile dyes due to their fastening properties on fabrics, preceded by azo dyes (Chaudhari et al., 2017). AQ dyes, which are typically prepared by nitration followed by a reduction reaction, contain the amino group. This generates nitrate residues that end up as effluents in wastewater (Chaudhari et al., 2017). AQ dyes are nondegradable in nature and prevent light from entering the water, thereby adversely affecting the aquatic flora and fauna (Berradi et al., 2019). Despite their prevalence, not many studies have been conducted on the degradation or treatment of AQ dyes (Routoula and Patwardhan, 2020).
Various treatment methods are available for AQ dye degradation, including photocatalytic oxidation (Zhang and Lu, 2018), ultrasonic catalytic oxidation (Samanta et al., 2018), ozone oxidation (Lovato et al., 2017), Fenton oxidation (Khataee et al., 2016), and microwave catalysis (Park et al., 2018). However, compared with these chemical methods, bioremediation techniques are ecofriendly, cost-effective, widely applicable, and highly preferable for the degradation of dyes present in industrial effluents (Paz et al.,2017). In typical biological treatments, decolorization through enzymatic degradation, biosorption, or a combination of both is carried out (Almeida and Corso, 2019). AQ dyes are degraded using microorganisms, such as fungi (Zhang et al., 2016; Šlosarčíková et al., 2017), actinomycetes (Linde et al., 2014), bacteria (Parmar and Shukla, 2018), and algae (Otto and Schlosser, 2014).
Fungi have non specific and complex enzyme systems and therefore show better degradation abilities than bacteria or algae. Many species of fungi synthesize extracellular ligninolytic enzymes, which have alow substrate specificity and can therefore be used in the biodegradation of aromatic compounds that have structural similarity to lignin (Tkaczyk and Kowalska, 2020). White rot fungi produce oxidoreductase enzymes such as laccase, lignin peroxidase (LiP), and manganese peroxidase (MnP). These enzymes can degrade toxic pollutants in wastewater such as polycyclic aromatic hydrocarbons, synthetic dyes, polychlorinated biphenyls, lignocelluloses, and phenols (Yanto et al., 2019, 2017; Hidayat and Yanto, 2018). Thus, an integrated review containing degrading fungi and their universal mechanisms of fungal AQ dye removal is required. In this review, AQ dye degradation using free and immobilized fungi along with their degradation mechanisms and key influencing factors is also discussed. This review provides valuable information regarding AQ dye wastewater management.
Structure and classification of AQ dyes
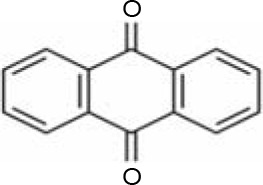
In AQ dyes, AQ chromophore groups comprising two carbonyl groups on both sides of a benzene ring (Table 1A) are present. Diverse AQ dyes can be produced by altering the substituents present (amino, hydroxyl, halogen, and sulfonic acid substituents). Furthermore, the color of AQ dyes depends on the location and properties of the substituents. Electron-accepting and electron-donating substituents show diverse effects, with the introduction of the former having a lower influence on color. However, electron-donating substituents (i.e., amino or substituted amino groups) show a significant impact on the color of dyes, with the color altering from blue to red, yellow, or purple (Table 1A–1D) (Duval et al., 2016). AQ dyes have been classified into four categories: 1) heterocyclic quinone dyes, such as Vat Yellow 28 (Table 1E); 2) AQ derivatives, such as Disperse Red 3B (Table 1F) and Acid Green 25; 3) heterocyclic anthrone dyes, such as Pigment Yellow 24 (Table 1G); and 4) fused ring anthrone dyes, such as Vat Green 1 (Table 1H). The color of AQ dyes varies greatly because of their substituents, and this is also the primary reason why the same microbial strains have different degradation efficiencies for different dyes.
Table 1
Basic structure of several anthraquinone dyes
Enzymatic degradation and detoxification of AQ dyes
Trametes hirsuta D7 is a strain of white rot fungus that has been newly isolated from the peat swamp forests of Riau, Indonesia. It secretes ligninolytic enzymes such as peroxidases (LiP and MnP) and laccases, which can effectively degrade several xenobiotic compounds including dyes (Hidayat and Yanto, 2018). Alam et al. (2021) carried out the degradation of three AQ dyes, Reactive Blue 4 (RB4), Remazol Brilliant Blue—R (RBBR), and Acid Blue 129 (AB129), by immobilizing T. hirsuta D7 fungal biomass in a light-expanded clay aggregate (LECA). UV absorbance data revealed that the efficiency of the degradation of RB4, RBBR, and AB129 was 90, 95, and 96%, respectively. High-resolution mass spectrometry was used to identify the fungal metabolites, and the degradation pathway of T. hirsuta was thus proposed.
Furthermore, it was observed using toxicity assays on human fibroblast cells that AQ dyes exhibit significantly high levels of toxicity (35, 40, and 34 in RB4, RBBR, and AB129, respectively) and reduced cell viability. However, wastewater treatment using immobilized fungal biomass effectively reduces toxicity and increases cell viability by upto 94%. Thus, T. hirsuta D7 immobilized in LECA effectively degrades AQ dyes. In this study, LECA was pretreated by steam activation at 800–900°C for 1 h. Ardiati et al. (2019) have previously reported the effectiveness of steam activation in enhancing dye removal. This technique helps in widening the microporosity of the carrier. In the present study, LECA removed 35% of dyes from wastewater. Several other studies have also explored the effect of adsorption by LECA and enzymatic degradation by T. hirsuta D7 in dye removal (Bahmanpour et al., 2017; Dordio and Carvalho, 2013). Thus, a combination of biodegradation and biosorption is the most suitable technique for the removal of toxic dyes from wastewater.
In a study by Pan et al. (2017), Aspergillus sp. XJ-2 removed six AQ dyes.The maximum removal of 93% was observed for Disperse Blue 2BLN (50 ppm) after 120 h of incubation. In addition, Deveci et al. (2004) reported the effective removal of the AQ dye RBBR (400 ppm). However, an increase in the dye concentration beyond the threshold limit inhibited this process (Yang et al., 2009). Since entrapped cells offer a higher exposure to the fungal biomass, thereby allowing multiple remediation cycles, they have an advantage over free cells or entrapped cells. Cell entrapment also helps in the removal of dyes even in the later cycles when laccase activity might have slowed down in the column (Przystas et al., 2018). Furthermore, entrapped fungal biomass helps it tolerate high concentrations of contaminants by increasing its enzymatic and physical contact with the pollutants (Lowet al., 2009; Papadopoulou et al., 2013).
Immobilized enzymes in AQ dye degradation
Jankowska et al. (2020) reported the use of electrospun poly(methyl methacrylate)/polyanilinefibers in the immobilization of laccase to remove RBBR dye (87% and 58% by adsorbed and covalently bonded laccase, respectively). Similarly, Couto et al. (2004) studied several carriers for the entrapment of T. hirsuta and found stainless steel to be the most suitable.
Zhao et al. explored (2012) the use of three myco valorization approaches, namely free-cell dependent, fungal entrapment using a column bioreactor, and enzymatic treatment using Myrothecium verrucaria, in the removal of AQ dyes. Glucose and peptone aid laccase production in M. verrucaria NF-08 (Hao et al., 2007; Gao et al., 2015). While studies on laccase produced by M. verrucaria have been reported, the activity of other dye-degrading enzymes like MnP and LiP produced by the strain was found in the available literature (Zhao et al., 2012, 2014; Podieiablonskaia et al., 2017). In a study by Cantele et al. (2017), phytotoxicity assays carried out after the simultaneous decolorization and submerged cultivation of AQ and azo dyes showed that nontoxic metabolites were formed.
There are multiple reports of bioremediation/bioremoval of dyes using laccase produced by fungal varieties such as Trametes and Myrothecium sp. (Hadibarata et al., 2012). SQ01 laccase has beenused for the decolorization of azo, triphenylmethane, and AQ dyes (Yang et al., 2009). Daassi et al. (2014) reported the biodegradation of AQ dyes using both free and immobilized laccase isolated from Coriolopsis gallica. In bioremediation experiments, fungal biomass has also been immobilized on various other support media such as washer, brush, grid, and sawdust (Przystaś et al., 2018). Therefore, finding the most feasible strategy considering efficiency, cost-effectiveness, and eco-friendliness is like searching a needle in a haystack.
Agrawal et al. (2020) used column bioreactors containing M. verrucaria ITCC-8447 in the removal of two AQ dyes from wastewater. They studied three approaches, and among them, the free-cell-dependent method removed more than 80% of the AQ violet R (AQVR) and Alizarin cyanine green (ACG) after 48 h at a temperature of 30± 2°C. However, enzymatic treatment removed more than 50% of the ACG and AQVR after 10 min. They used Scotch-Brite® (SB) as a synthetic support for the entrapment of fungal biomass and Luffa cylindrica (LC) as a natural support. SB-ACG successfully removed more than 80% of the dyes, whereas LC-ACG removed more than 60% of the dyes by the sixth remediation cycle. Regeneration of the column enhanced its dye-removing efficiency, thus increasing reusability. Fourier transform infrared spectroscopy, quadrupole time of flight liquid chromatography – mass spectrometry (Q-TOF LCMS), and X-ray diffraction showed that ACG and AQVR were completely degraded, with laccase playing a key role. Phytotoxicity assays performed on the degraded metabolites of the dyes showed that they were significantly less toxic than the parent dye.
In another study, in situ free cell approach and immobilization on L. cylindrica were applied to decolorize AQVR using fungal biomass of Stropharia sp. ITCC-8422 (Agrawal and Verma, 2019b). Although all these methods showed effective decolorization, the usage of column bioreactors resulted in more than 55% of AQVR removal in the seventh cycle. Immobilization of the fungal biomass showed effective decolorization of AQVR. More than 80% of the decolorization was observed in the column bioreactor upto the third cycle, whereas upto the seventh cycle, decolorization reduced to more than 55%. Regeneration of the column reactor improved the decolorization rate and may be used for multiple cycles. However, the immobilized fungal biomass or natural support showed no significant adsorption potential. More than 80% of the decolorization of AQVR (100 ppm) was attributable to crude yellow laccase (pH 9, 40°C, after 10 min). The degraded products (with low molecular weights of m/z = 146.0607 and 130.064) were then identified using Q-TOF LCMS, and then, a degradation pathway of fungal biomass was proposed. The treated and untreated dyes were also examined using spectral analyses, and toxicity assays were carried out on the degraded and parent dyes. The degraded dyes were significantly less toxic (Agrawal et al., 2019b).
Hadibarata et al. (2012) performed the decolorization of AQ dyes, in which 90% of the decolorization was observed at concentrations lesser than or equal to 300 ppm, whereas only 75% was observed when the concentration of dyes was between 400 and 500 ppm.
Despite the importance and extensive usage of AQ dyes, only a few studies have investigated their degradation mechanisms. Different techniques available for the decolorization of wastewater reflect the vastness of the issue. According to the available literature, only a few fungal and bacterial strains with AQ degradation potential have been isolated to date (Hsu et al., 2012; Champagne and Ramsay, 2010). Therefore, there is a high demand for research on novel, eco-friendly, and cost-effective wastewater treatment processes, especially for wastewater containing toxic textile effluents such as AQ dyes.
Among the various techniques used in the biological treatment of wastewater, immobilization of culture biomass has been reported to be the most suitable as it prevents the accumulation of toxic end products and has higher efficiency (Przystaś et al., 2018). Several carrier materials with dye removal abilities have also been explored. However, the long-term usage of fungi in bioreactors under nonsterile conditions remains to be researched extensively.
Conclusions and outlook
A thorough and systematic study of the treatment of AQ dyes has drawn interest in “green technology” to develop more sustainable and efficient treatment methods for AQ dyes, such as biological treatments and valorization. Several microbial activities based on enzymatic activities can be carried out at extreme dye concentrations, with large ranges of pH values, temperature, and salinity. High-specificity fungal enzymes can decompose high concentrations of persistent AQ dyes extremely quickly. Furthermore, effective decolorization of real textile effluents and a significant decline in toxicity can be achieved using laccase. The superior properties of fungal enzymes have made them potential candidates for color removal of diverse textile dyes from wastewater in a sustainable, cheap, and environmental-friendly manner. The decolorization potential of immobilized cells is more stable than that of free cells after repetitive use. The outcome of these studies justifies the use of immobilized culture biomass as a primary alternative in AQ dye degradation. These immobilized fungal strains are more stress resistant with improved adaptability and consistency than free strains. Moreover, engineering the genome of the fungus to produce improved fungal strains can also result in remarkably adaptive and upgraded myco remediation techniques for extremely recalcitrant dyes.
In addition, methodical expansion in this field necessitates active collaborations between genetic engineers, chemical engineers, chemists, and biologists to overcome the technological hurdles and strengthen the perceptive of AQ myco remediation processes in terms of stability, kinetics, and operational capabilities. These collective endeavors will further improve the application of myco remediation methodologies in the control of AQ contamination and related fields.