Introduction
Conventionally, farmers have long used synthetic fertilizers to improve soil nutrients. However, excessive application of these chemicals leads to the accumulation of contaminants in agricultural soils (Lu et al., 2018), and consequently, significant damages to important crops have been recorded (Herren et al., 2020; Musa and Ikhajiagbe, 2021). For example, in ferruginous soil, which is characterized by high iron content, acidic pH, and phosphorus deficiency (Yu et al., 2016), the application of phosphorus-containing chemicals has further complicated the nutrient levels because phosphorus forms complexes with iron (Fe3+) in acidic soils, thus resulting in a ferrous phosphate (FePO) soil (Satyaprakash et al., 2017; Kumar et al., 2018). These processes make phosphorus not available for plants and increase soil acidity, which restricts the proper growth and yield of crops (Walpola and Yoon, 2012). Since a high proportion (45.2%) of the Earth’s surface is covered by ferruginous soil and due to its high presence in some southern states of Nigeria such as Edo State (Doyou et al., 2017), it is important to develop an alternative strategy that can sustainably promote high crop yields under ferruginous soil conditions.
Recently, the use of plant-growth-promoting (PGP) bacteria has proven effective and sustainable in improving the growth properties of plants (Shen et al., 2016; Kuan et al., 2016; Suleman et al., 2018). In particular, PGP bacteria have improved the availability of important soil nutrients through symbiotic N2 fixation and promoted the rate of secretion of phytohormones such as indole acetic acid (IAA), cytokinins, and gibberellins (Shen et al., 2016). Backer et al. (2018) have observed an improvement in the physiological parameters of sweet pepper (Capsicum annum ) as a response to inoculation with PGP bacteria. Several studies have reported improved root length, surface area, and general growth of chick pea (Cicer arietinum ) and pigeon pea (Cajanus cajan ) under zinc and copper concentrations after the application of PGP bacteria (species of Enterobacter ) (Ikhajiagbe and Musa, 2020a; Rizwan et al., 2018; Gopalakrishnan et al., 2016). However, in ferruginous soil with high iron and low phosphorus levels, the application of PGP bacteria alone may not yield the desired results because they may not withstand the acidity and iron toxicity of the soil (Dobermann and Fairhurst, 2000). Hence, previous studies have suggested the inoculation of phosphate-solubilizing bacteria (PSB) for the effective chelation of soil phosphorus to bioavailable phosphorus for plant use (Wang et al., 2014). Several strategies for the inoculation of bacteria to improve growth properties have been reported in the literature (Rizwan et al., 2018; Gopalakrishnan et al., 2016). Previous studies have attributed the successful application of PGP bacteria to the techniques used to inoculate the bacteria with the host plant (Mahmood et al., 2016; Gopalakrishnan et al., 2016). Musa and Ikhajiagbe (2021) have previously investigated foliar spray and rhizo-inoculation by introducing microorganisms into the root of plants to stimulate plant growth. However, these techniques have certain limitations with respect to efficiency in seed emergence and germination studies, as reported by Mahmood et al. (2016). Although there are several other strategies for PGP bacteria inoculation into the host plant, seed bio-priming has been reported as one of the most effective ways to achieve seed surface bacterial inoculation. Bio-priming involves soaking seeds using living bacterial inoculum for a specific duration to allow bacterial imbibition into the seed (Ashraf and Foolad, 2005). This process initiates some physiological responses in seeds that result in rapid colonization and acclimatization under controllable conditions (Mahmood et al., 2016). As reported by Basavaraj et al. (2019), bio-priming provides sustainable conditions for bacteria to multiply rapidly and proliferate in spermospheres, leading to improvements in seed viability germination, germination speed, seed vigor, germination time, and growth and yield of plants (Ikhajigbe and Musa, 2020a; Rajendra et al., 2016). Bio primed seeds have been reported to be efficient in improving the germinability and morphological performance of seeds under stressful environmental conditions (Mirshekari et al., 2012). Considering the effectiveness of bio-priming in PGP bacteria inoculation, this study aimed to investigate the possibilities of bio-priming rice (Oryza sativa L.) seeds with PGP bacteria with PSB capabilities to improve the growth properties of rice under ferruginous conditions.
The following PGP bacteria with PSB were used in this study: Bacillus cereus strain GGBSU-1, Klebsiella variicola strain AUH-KAM-9, and Proteus mirabilis strain TL14-1, which were previously isolated and identified by Musa and Ikhajiagbe (2020a) using 16S rDNA sequencing from ferruginous and humus soils in Benin City, Nigeria. These PSB isolates have proven to be efficient in solubilizing insoluble phosphate in soils (Musa and Ikhajiagbe, 2020a; Addendum 1) and show PGP capabilities by releasing IAA and siderophores (Musa and Ikhajiagbe, 2020a; Addendum 2 and 3). Furthermore, they have significantly improved the germination and yield parameters in rice in vitro (Musa and Ikhajiagbe, 2021). In the present study, another sustainable strategy of improvement in agricultural productivity and food security under ferruginous ultisol conditions was presented.
Materials and methods
Experimental soil preparation
The experiment was conducted at the experimental garden of the Department of Biology and Forensic Science, Admiralty University of Nigeria, Delta State, Nigeria. Ferruginous soils previously obtained by Musa and Ikhajiagbe (2020b) from six locations around Benin City, Edo State, Nigeria, were pooled to obtain a composite sample. The composite ferruginous soil was prepared in 12 sections in experimental bowls (60 × 25 cm). Each section was prepared in five replicates.
Soil physiochemical parameters
The ferruginous soil samples were air-dried at 22–25°C and then analyzed for soil organic matter levels, soil available phosphorus, cation exchange capacity, pH, total nitrogen content, organic carbon (OC) content, exchangeable acidity, available potassium, available micronutrients such as sodium (Na) and aluminum (Al), electrical conductivity, soil texture class, and maximum water-holding capacity, following the protocols reported by Musa and Ikhajiagbe (2020a). The iron content of the soil was analyzed following the method of Cheng et al. (2013) using concentrated perchloric acid to digest the soil sample and titrating it with versenate solution, as described in Ikhajiagbe and Musa (2020a).
Bacterial species
In this study, the three PSB, previously isolated by Musa and Ikhajiagbe (2021) from ferruginous ultisol (FU) and humus soil from Benin City, were prepared in stock cultures. These PSB were previously identified using 16S rDNA sequencing after biochemical tests involving catalase, indole, citrate, nitrogen-fixing activities; bromothymol blue test; and pH tolerance level test with hydrochloric acid, following the method of (Mondala et al. (2016). PGP capabilities of the isolates were determined by IAA and siderophore production following the protocols of Gupta et al. (2012b) and Balkar (2013), respectively, and as reported in Musa and Ikhajiagbe (2021). Meanwhile, phosphate-solubilizing capabilities were analyzed using Pikovskaya’s media as reported by Musa and Ikhajiagbe (2021) and (Supplementary figure 1, 2 and 3).
Preparation of inoculum
The pure PSB with PGP traits (B. cereus strain GGBSU-1, K. variicola strain AUH-KAM-9, and P. mirabilis strain TL14-1) were prepared by streaking the bacteria onto agar plates (Yadav et al., 2015) and incubating them at 28°C for 48 h. After 48 h of growth, the isolates were inoculated in nutrient broth and then prepared in 0.5 McFarland Standard with Cat. no (TM50) to standardize the approximate number of bacteria in the suspension. Then, 500 ml of each bacteria isolate was prepared to obtain an average microbial suspension of 1.5 × 108 CFU/ml.
Biosafety analysis of the isolated PSB
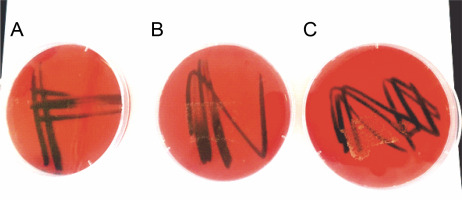
Since some strains of bacteria can be harmful to humans (Muhamad et al., 2020), biosafety analysis of the isolated PSB was carried out using a blood agar medium (Russell et al., 2006). The three PSB isolates were poured on blood agar plates of 5% blood. The agar was incubated at 40°C for 2 days. Hemolysis of red blood cells was assessed by the formation of a clear zone around colonies (β-hemolysis) or greenish coloration (α-hemolysis), whereas no clear holo-zone indicated γ-hemolysis.
Rice seed bio-priming
Seeds of rice variety (FARO 44) previously obtained from the Center for Dryland Agriculture, Bayero University, Kano, Nigeria, were used in this study. The seeds were tested for viability (AOSA, 2000) and surface sterilized with 70% ethanol following the protocol by Sameer and Nabeel (2016). The sterilized seeds were dried at room temperature for 3 h and then sown onto 45 presterilized Petri dishes (9c diameter) at the rate of 10 seeds per petri dish, which were lined with Whatman filter paper. Of the prepared 500 ml bacteria inoculum of each bacteria, 10 ml was introduced into each Petri dish and studied for 3, 12, and 24 h to allow bacteria colonization. The control was 10 ml of distilled water. The setup was kept between 25 and 26.6°C as described by Etesami et al. (2014). The three bacteria were coded as follows: A – B. cereus strain GGBSU-1, B – P. mirabilis strain TL14-1, and C – K. variicola strain AUH-KAM-9, whereas the control containing 10 ml of distilled water was coded FD in FU.
Sowing of rice seeds
Three different bio-priming setups were transplanted after 3, 12, and 24 h into the prepared experimental bowls containing the assayed ferruginous soils (Table 1). Each of the bacteria-primed seeds was transplanted into 12 ferruginous soil sections at a rate of 10 seeds per pot. The setup was made in an open (no shade) environment and as such relied on rainfall. However, soil moisture was always maintained periodically following the methods of the United States Department of Agriculture (USDA, 2010). This setup was weeded every 2 days and maintained for 15 weeks after priming.
Table 1
Physical and chemical parameters of the experimental non-ferruginous (control) and ferruginous soils
Rice seed germination bioassay
Germination parameters are highly effective in determining the influence of bacterial priming on seeds. They were analyzed in two ways. First, the time at which the first coleoptile (primary leaf) appeared was determined; this was monitored in vitro using 20 seeds per inoculum at different bio-priming periods and observed by monitoring the setup every 6 h at 25°C in a dark room at the Department of Plant Biology and Biotechnology, University of Benin Postgraduate Research laboratory (ISTA, 2005).
The second was germination percentage, which was monitored in vivo after rice seeds were transplanted into soils. Twenty seeds per inoculum were used. Germination was considered as the period at which the first cotyledon protruded visibly from the soil, which was calculated as follows (ISTA, 2005):
Bacterial colonization capabilities of the three PSB with PGP traits in rice seeds after 24 h of bio-priming were observed using electron microscopy. Eight endosperm samples (1 cm) were prepared aseptically and kept in vials for each treatment. Electron microscopy analysis was conducted following the method of Muhamad et al. (2020), using a Jeol JSM-6400 scanning electron microscope.
Morphological properties of test plants
Morphological parameters related to the growth and yield of rice were investigated. Fresh shoot length, length of internodes, and length of the first leaf were calculated in centimeters using a transparent ruler mounted on a white calibrated paper throughout the study. To determine the dry shoot length (cm), seedlings were air-dried for 24 h and then weighed at 2-week intervals from day 3 to 16 weeks after bio-priming. The length of internodes (cm) was measured weekly from the coleoptile to the first node using a sample of five seedlings from all treatments. The average time required for the formation of one leaf was determined as time per leaf formation (T /LF ) following the method of Dobermann and Fairhurst (2000):
where T / LF is the time taken for the formation of one leaf, nl is the number of leaves per unit time, n is the number of periods, and t is the time at which the parameter was measured.
Physiological parameters of test plants
To analyze the influence of bacterial bio-priming on the growth and yield of the rice plant, physiological parameters were investigated. The total soluble sugar (TSS) content of fresh leaves was estimated from week 5 to week 14, with 2-week intervals, by oven drying the tallest leaf at 70°C for 24 h as described by Nelson (1944), with slight modifications (Sankar and Selvaraju, 2015). Growth enzymes such as alpha amylase (AA) of the growing plants were determined on similar days as TSS following the method of Lowry et al. (1951) at a pH of 7.5. Terpenoid and lycopene contents of fresh leaves were determined on similar days as AA following the method of Moran (1982). The chlorophyll content index (CCI) of old and fresh leaves was determined via a non-destructive method using an Apogee chlorophyll concentration meter. The CCI was measured as the average of the mesocotyl, mid-seedling, and top seedling on similar days as AA. Chlorophyll a and chlorophyll b contents were determined following the methods of Dobermann and Fairhurst (2000) and Maxwell and Johnson (2000).
Biomass parameters of test plants
Leaf area (cm2) was calculated using an android application (Leaf-IT) following the method of Julian et al. (2017) from week 5 to week 14, with 2-week intervals. The number of leaves was measured by counting every week till harvest. Leaf tip chlorosis and necrosis were measured as the percentage of the total number of leaves produced by plants with significant levels of chlorosis and necrosis. This was pre-decided following reports from Ikhajiagbe and Musa (2020a) from week 3 to week 14, with 2-week intervals. The weight of fresh leaves (g) was measured using an analytical weighing balance on similar days as AA.
Root growth parameters of test plants
Fresh root length was calculated in centimeters using a transparent ruler mounted on a white calibrated paper throughout the study. To determine the dry root length (cm), seedlings were air-dried for 24 h, and the roots were measured from day 3 to 16 weeks after bio-priming. Meanwhile, the number of secondary roots was carefully observed and counted daily.
Rice harvest properties
Other rice yield properties such as the time at which maturity was achieved, the number of tillers, the number of reproductive tillers, the number of panicles, straw yield, the number of seeds per panicle, the weight of rice panicles, the weight of peduncles without rice, the weight of 100 grains, and plant tissue water were calculated on the harvest day.
Statistical analysis
Data obtained from the analysis were expressed in means and standard errors of five replicates. Data were analyzed using two-way analysis of variance using GENSTAT (8th edition). Where significant p-values were obtained, differences between the means were separated using the Student–Newman–Keuls test (Alika, 2006). The ferruginous soil used in the experiment was homogenized.
Results
Soil physicochemical properties
The physicochemical properties of the ferruginous soil used in this study are presented in Table 1. Results revealed that the ferruginous soil was acidic with a pH of 5.01 and had a total soil OC content of 7.31%. The iron content was high (200.67 mg/kg), whereas the available phosphorus level was low (8.01 mg/kg). The textural class was observed to be loamy-sandy, with a poor waterholding capacity (86.89%).
Biosafety assay of the isolated PSB
Results of the PSB biosafety test using blood agar showed that the three isolated PSB did not show any hemolytic activity because no detectable clear zones (γ-hemolysis) were observed (Fig. 1).
Germination assay and seed colonization of the three PSB
To investigate the influence of the three PSB on germination parameters of rice seeds at the three different bio-priming durations, a germination bioassay was carried out. The coleoptile did not appear in the 3-h bio-priming setup. However, the first coleoptile appeared at 12 h in Bacillus-primed seeds (FA), followed by Proteus-primed seeds (FB) at 18 h, whereas the Klebsiella-primed seeds (FC) appeared at 24 h (Table 2). No sign of coleoptile appearance was observed in the ferruginous soil without PSB inoculation (FD) throughout the 24-h bio-priming experiment (Fig. 2). After transplanting, FA transplanted after 12 and 24 h of priming (FA12 and FA24) were the first to achieve complete germination (100%) at 2 days after transplanting (DAT), followed by PPS (FB12) at 4 DAT (Table 2). A significant delay (P > 0.5) was observed in the germination percentage of FD on all assayed days. The germination results indicated the highest positive influence of FA (94%) compared with FB (91%) and FC (88.3%), whereas FD showed the lowest influence (29%).
Table 2
Germination parameters of rice seeds after 24 h of bio-priming with three phosphate-solubilizing bacteria isolates
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); all data were expressed as mean and standard error of five replicates; DAT – day after transplant, APC – appearance of coleoptile; FA3 – ferruginous soil bio-primed with Bacillus sp. for 3 h, FB3 – ferruginous soil bio-primed with Proteus sp. for 3 h, FC3 – ferruginous soil bio-primed with Klebsiella sp. for 3 h, FD – ferruginous soil without bio-priming; the number after each sample signifies the duration of priming
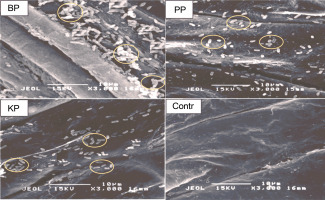
To confirm the effective colonization of the three PSB on rice seeds in vitro, the tin endosperm section of bioprimed rice seeds and the control was observed using SEM after 24 h of bio-priming. Images of endosperm sections (Fig. 3) viewed under 3000 × magnification showed that the three PSB successfully colonized and proliferated on the endosperm at different cell proliferation levels. BPS showed the highest bacterial population, whereas the control showed no bacterial population.
Fig. 3
Images of rice endosperm sections showing in situ colonization and acclimatization of phosphate-solubilizing bacteria (indicated by oval) under scanning electron microscopy; BPS – Bacillus cereus strain GGBSU-1-primed seeds, PPS – Proteus mirabilis strain TL14-1-primed seeds, KPS – Klebsiella variicola strain AUH-KAM-9-primed seeds, Control – rice seeds without PSB priming
Effect of bioprimed PSB on rice morphological parameters
Since SEM imaging (Fig. 3) indicated bacterial colonization as a result of bio-priming, a seedling pot experiment was carried out to evaluate the possibilities of the PSB to solubilize phosphate in ferruginous soil to improve rice plant morphology. Differences (Figs. 4–6, Table 3 and Addendum 3) were observed in rice seedling morphological parameters between PSB bioprimed seeds in ferruginous soils and the control (ferruginous soil without PSB priming). Compared with the ferruginous soil without priming, a significant increase (P < 0.05) in the fresh shoot length (Fig. 4) of all PSB-bioprimed seeds was observed. FA12 showed the highest growth performance on all assayed days, whereas FD24 showed the least morphological properties. Dry shoot length, length of the first leaf, and length of internodes were all improved in PSB-bioprimed seeds compared with nonprimed seeds (Table 3). At 8WAT, delayed shoot length and lower length of internodes were observed in all the samples. Furthermore, bioprimed seeds took significantly lower time (33% low) for single leaf formation compared with nonprimed seeds (Table 3).
Fig. 4
Fresh shoot length of rice from 3 days after priming to 14 weeks after priming; DAT – days after transplanting, WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control –rice seeds without PSB priming
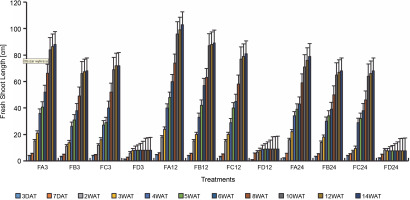
Fig. 5
Length of the first rice leaf 3 days after bio-priming to 14 weeks after bio-priming; WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
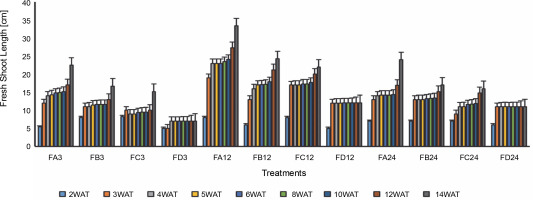
Fig. 6
Length of rice internodes 3 days after bio-priming to 14 weeks after bio-priming; DAT – WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
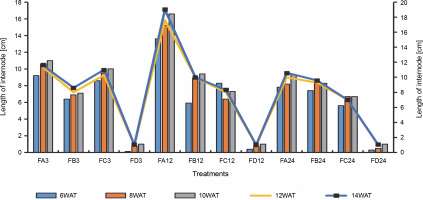
Table 3
Response of dry shoot length and time taken for rice leaf formation to phosphate-solubilizing bacteria bio-priming
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; WAT – week after transplant, T/LF – time taken for the formation of one leaf, FA3 – ferruginous soil bioprimed with Bacillus sp. for 3 h, FB3 – ferruginous soil bioprimed with Proteus sp. for 3 h, FC3 – ferruginous soil bioprimed with Klebsiella sp. for 3 h, FD – ferruginous soil without bio-priming; the number after each sample signifies the duration of priming
Effect of PSB bio-priming on rice physiological parameters
Since terpenoids signal biotic and abiotic stress in plants, terpenoid levels were assayed to observe whether ferruginous soils signal either biotic or abiotic stress and how bio-priming reduces the possible stress in rice plants. As shown in Figure 7, high terpenoid levels were observed in non primed rice plants irrespective of the priming duration, whereas PSB-bioprimed rice plants showed lower levels of terpenoids (Fig. 8). A significant increase in lycopene levels was observed in bioprimed rice plants compared with nonprimed plants (Fig. 8). Significant differences (Figs. 9–11) were observed in the TSS content, AA levels, and CCI between bioprimed rice plants and nonprimed plants. BPS showed higher (45%) TSS content and AA levels than KPS and PPS. Generally, the CCI of old and new leaves showed similar trends as in TSS and AA.
Fig. 7
Terpenoid levels of rice plants from 3 days after priming to 14 weeks after priming; WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
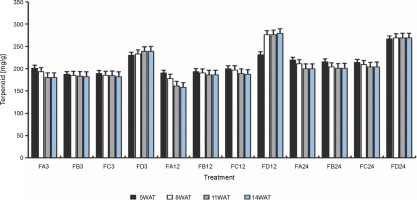
Fig. 8
Lycopene levels of rice plants from 3 days after priming to 14 weeks after priming; WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
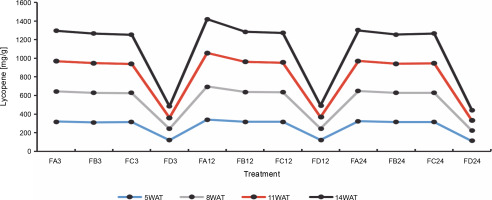
Fig. 9
Total soluble sugar levels of rice plants from 3 days after priming to 14 weeks after priming; WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
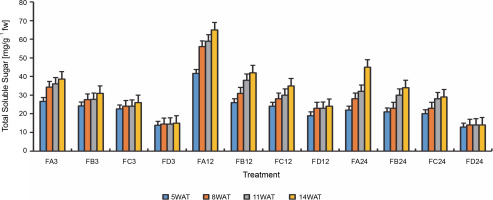
Fig. 10
Alpha amylase levels of rice plants from 3 days after priming to 14 weeks after priming; WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
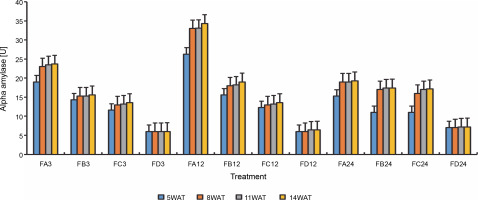
Fig. 11
Chlorophyll content index of old and new leaves of rice plants from 3 days after priming to 14 weeks after priming; OL – old leaves, NL – new leaves, WAT – weeks after transplanting, FA – Bacillus cereus strain GGBSU-1-primed seeds, FB – Proteus mirabilis strain TL14-1-primed seeds, FC – Klebsiella variicola strain AUH-KAM-9-primed seeds, FD – Control – rice seeds without phosphate-solubilizing bacteria priming
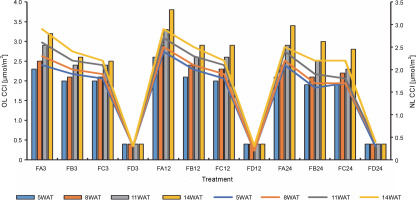
The chlorophyll content of rice leaves from 5WAT to 16WAT, as shown in Table 4, showed that there were significant changes in the chlorophyll pattern. Nonprimed rice plants showed a lower chlorophyll-a content than bioprimed plants. A similar trend was observed in the chlorophyll b content. Generally, BPS showed the highest chlorophyll contents.
Table 4
Chlorophyll a and chlorophyll b contents of rice plants after bio-priming
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; DAT – day after transplant, FA3 – ferruginous soil inoculated with Bacillus sp. at 3 h of bio-priming, FB3 – ferruginous soil inoculated with Proteus sp. at 3 h of bio-priming, FC3 – ferruginous soil inoculated with Klebsiella sp. at 3 h of biopriming, FD – ferruginous soil without inoculation at 3 h of bio-priming; the number after each sample signifies the duration of priming
Effect of PSB bio-priming on rice biomass parameters
A significant increase in biomass was observed in bioprimed rice plants than in nonprimed plants. FA showed higher weight of fresh plants and leaf area (Table 5) at all durations across all assayed days than FB and FC. Priming with B. cereus strain GGBSU-1 (FA12) for 12 h showed better rice biomass properties such as weight of fresh plant (5.2 g-1) and largest leaf area (4.8 cm2) than FB and FC (Table 5). In nonprimed plants, a higher percentage of leaves showed signs of chlorosis and necrosis at the leaf tip (80%), whereas only 12% of leaves showed signs of leaf tip necrosis in BPS at 12 h (Table 6).
Table 5
Weight of fresh plant and area of rice leaf after bio-priming
[i] Results with similar alphabets superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; WAT – weeks after transplant, FA3 – ferruginous soil inoculated with Bacillus sp. at 3 h bio-priming, FB3 – ferruginous soil inoculated with Proteus sp. at 3 h of bio-priming, FC3 – ferruginous soil inoculated with Klebsiella sp. at 3 h bio-priming, FD – ferruginous soil without inoculation at 3 h of bio-priming; the number after each sample signifies the duration of priming
Table 6
The number of rice leaves and the number of leaves with signs of necrosis and chlorosis at the tip after bio-priming
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; WAT – weeks after transplant, FA3 – ferruginous soil inoculated with Bacillus sp. at 3 h of bio-priming, FB3 – ferruginous soil inoculated with Proteus sp. at 3 h of bio-priming, FC3 – ferruginous soil inoculated with Klebsiella sp. at 3 h of bio-priming, FD – ferruginous soil without inoculation at 3 h of bio-priming; the number after each sample signifies the duration of priming
Effect of PSB bio-priming on rice root parameters
The length of fresh and dry roots (Table 7 and Table 8) was improved in FA12 than in FB and FC. The number of secondary roots (Table 7) was increased in FA, FB, and FC. A higher number of secondary roots (10) were observed in ferruginous soil without inoculation at 3 weeks after transplanting (3WAT) at all biopriming durations. However, this was reduced significantly by 40% on 6WAT and 8WAT.
Table 7
Response of fresh root length and number of secondary roots of rice plants to phosphate-solubilizing bacteria bio-priming
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; WAT – week after transplant, FA3 – ferruginous soil bioprimed with Bacillus sp. for 3 h, FB3 – ferruginous soil bioprimed with Proteus sp. for 3 h, FC3 – ferruginous soil bioprimed with Klebsiella sp. for 3 h, FD – ferruginous soil without bio-priming; the number after each sample signifies the duration of priming
Table 8
Response of dry root length of rice plants to phosphate-solubilizing bacteria bio-priming
[i] Results with similar alphabets as superscripts on the same column did not differ from each other (P > 0.05); results were expressed as mean and standard error of five replicates; WAT – week after transplant, FA3 – ferruginous soil bioprimed with Bacillus sp. for 3 h, FB3 – ferruginous soil bioprimed with Proteus sp. for 3 h, FC3 – ferruginous soil bioprimed with Klebsiella sp. for 3 h, FD – ferruginous soil without bio-priming; the number after each sample signifies the duration of priming
General growth characteristics of test plants
Bioprimed rice seeds achieved maturity within 11 weeks after bio-priming. However, for plants grown on ferruginous soil without PSB priming (FD), growth and yield seized in the eighth week after priming (Fig. 12).
Fig. 12
Growth performance of rice plants 8 weeks after biopriming; FA – ferruginous soil inoculated with Bacillus sp., FB – ferruginous soil inoculated with Proteus sp., FC – ferruginous soil inoculated with Klebsiella sp., FD – ferruginous soil without inoculation
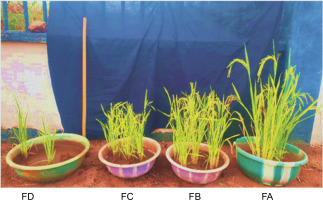
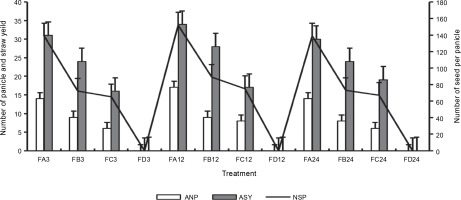
Figure 13 presents the number of tillers and reproductive tillers observed on the harvest day. Bio-priming of rice seeds significantly improved the number of tillers and the number of reproductive tillers in rice plants at harvest. FA showed a higher number of tillers (72) and reproductive tillers (46) after 24 h of priming than FC (60 and 32) and FB (62 and 32). Rice plants that were exposed to 12 h of priming showed the highest growth yield properties at harvest. Rice plants grown on ferruginous soil without priming stopped growing before tillers appeared. Similarly, PSB-primed plants showed a higher number of panicles per plant and seeds per panicle (Fig. 14), whereas rice plants grown on ferruginous soil without bio-priming did not survive till this stage.
Fig. 13
Rice harvest parameters; FA – ferruginous soil inoculated with Bacillus sp., FB – ferruginous soil inoculated with Proteus sp., FC – ferruginous soil inoculated with Klebsiella sp., FD – ferruginous soil without inoculation; the number after each sample signifies the priming duration
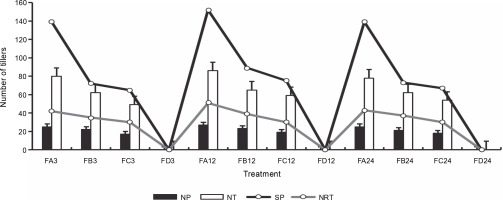
Fig. 14
Rice panicle parameters at harvest; ANP – number of panicles, ASY – straw yield, NSP – number of seeds per panicle, FA–FC – ferruginous soil under the bio-priming experiment with A, B, and C, respectively, A – Bacillus cereus strain GGBSU-1, B – Proteus mirabilis strain TL14-1, C – Klebsiella variicola strain AUH-KAM-9
Discussion
The possibilities to improve the growth parameters of rice plants through bio-priming with PSB under FU conditions were analyzed. FU has high acidity, high iron levels, low bioavailable phosphorus, and low water-holding capacity (Musa and Ikhajiagbe, 2020a). The physicochemical parameters of the ferruginous soil used in the present study are consistent with the properties described above (Table 1). Musa and Ikhajigbe (2021) and Wang et al. (2014) regarded such soil conditions as those with poor growing conditions because of their ferruginicity. Since the present study relied on bio-priming of rice seeds with PSB, biosafety analysis of the bacteria was conducted, which indicated that the bacteria were not pathogenic to humans and therefore safe for further plant studies. This is consistent with the study of Muhamad et al. (2020), who confirmed the hemolysis of certain phosphorus- and potassium-solubilizing Enterobacter sp. as inoculants in crop improvement.
The rapid coleoptile appearance observed in PSB-bioprimed rice seeds, compared with nonprimed seeds, proved the effectiveness of bio-priming in improving germination properties. This may be a result of the PSB’s ability to stimulate certain enzymes such as α-amylase, which improve coleoptile growth (Setter and Waters, 2003), thus increasing the chances of survival for the growing seedling (Huang et al., 2005). Furthermore, the rapid coleoptile appearance resulted in the early germination of bioprimed seeds, compared with nonprime seeds, 2 days after seedling transplant. This finding is consistent with an in vitro plate bioassay carried out by Suleman et al. (2018), who reported that Enterobacter spp. (MS32P) increased the germination properties of wheat. Similarly, muskmelon seed bio-priming with strains of endophytic bacteria resulted in the promotion of hypocotyl and radicle growth (Duan et al., 2013). Importantly, 12 h of rice seed priming with the bacterial isolates proved more effective than priming for 3 and 24 h. This is consistent with the study by Ananthi et al. (2014), who investigated the effects of different durations of bio-priming of chili seeds with Trichoderma viride and observed that bio-priming for 12 h was the most effective duration, as confirmed by the improved germination percentage and germination rate. This may be a result of the high bacterial population in rice seeds at 24 h, and since the bacteria need energy for their growth and multiplication, they may affect the seed energy efficiency (Basavaraj et al., 2019). As reported by Anitha et al. (2013), germination and enhanced seedling establishments can be achieved through seed biopriming with PGP bacteria.
Since bio-priming with PSB possessing PGP capabilities can change the seed microbiome and lead to root colonization in soils (Gupta et al., 2012a), effective bacteria colonization in rice seed endosperm was observed using SEM, where a large number of bacterial cells were detected on the endosperm. The results showed that these PSBs are motile, can easily proliferate within the rhizospheric environment, and can influence the Fe–P bond (Sachdev et al., 2009).
During the rice growth phases in ferruginous soil, improved morphological parameters such as shoot length and length of internodes observed in bio-primed rice seeds compared with nonprimed rice seeds indicated the potential of the PSB in improving the growth parameters of rice plants, even under ferruginous conditions. Rice seeds primed with Bacillus sp. for 12 h showed the highest growth influence even under ferruginous soil conditions (Table 1). Dobermann and Fairhurst (2000) described ferruginous soil as a soil that negatively impairs plant growth and yield by affecting plant metabolic processes, as observed in rice seedlings. As reported by Sharifi (2011), a significant increase in crop growth, dry matter accumulation, and grain yield was observed in maize when seeds were bioprimed with different strains of Azotobacter and Azospirillum spp. Priming soybean seeds with Trichoderma harzianum, T. viride, and Bacillus subtilis resulted in a significant increase in plant height, shoot weight, number of branches, and resistance against root rot disease, as well as seedling protection against soil borne infections (Mona et al., 2017). Suleman et al. (2018) highlighted a direct association between the bacterial pathway for P solubilization and the secretion of multiple cellular organic acids; hence, the low-molecular-weight organic matter produced by the PSB may lower the soil pH and chelate the Fe–P bond, thereby improving P availability, which in turn improves soil properties (Musa and Ikhajiagbe, 2020; Dobermann and Fairhurst, 2000). The high number of secondary roots observed in the non-primed seedlings, along with the lowest root length, may indicate nutrient deficiencies. Plants show different responses to different specific nutrient deficiencies; moreover, the responses can vary between species. An example of such a response is the secretion of metabolites such as terpenoid (Musa and ikhajiagbe, 2021; Esha et al., 2017). As reported by Jones and Ljung (2012), the most common changes in plants due to nutrient deficiencies are the inhibition of primary root growth (often associated with P deficiency) and the increase in the density of secondary roots (often associated with P deficiency and Fe toxicity). The high terpenoid content observed in non-primed seedlings may be associated with the poor ability of the ferruginous soil to support plant growth.
The higher CCI observed in the bio-primed seedlings showed a positive influence of PSB compared with nonprimed seedlings. Since nutrient availability is considered a limiting step in plant growth and nutrition (Rodríguez and Fraga, 2009), this suggests a significant contribution of the PSB in promoting the photosynthetic properties and yield of rice plants. Kennedy et al. (2004) reported that a wide range of PGP bacteria can influence the photosynthetic signals of plants, resulting in an improved grain yield and vegetative growth. Furthermore, a recent review of bio-priming techniques validated that the major microbial contributions toward enhanced plant productivity included, but were not limited to, N2 fixation, P solubilization, secretion of PGP hormones, secretion of beneficial organic acids, enhancement of nutrient uptake by the rhizosphere, and iron chelation (Ikhajiagbe and Musa, 2020b; Mahmood and Kataoka, 2018). All these parameters were observed in the present study. Rice seedlings bio- for 12 h showed the most improved physiological properties, indicating the influence of priming duration on rice growth. Duration of priming and water potential of the priming agents are considered factors influencing priming (Ashraf and Foolad, 2005; Abdulrahmani et al., 2007).
Evidence from the analysis of rice plant biomass parameters suggests a significant contribution of the PSB to improving the weight, leaf area, the number of leaves, and leaf health of rice plants compared with nonprimed rice plants (Supplementary figure 4). This indicated the growth-promoting capabilities of the PSB isolates and their ability to solubilize P in soil and make it bioavailable because P is considered a limiting nutrient for rice plants (Doberman and Fairhurst, 2000). The results of the present study are consistent with those of Gholami et al. (2009), who observed that stem area, leaf area, and total height of maize seedlings were increased after bio-priming of maize seeds with PGP bacterial isolates in a laboratory experiment. In the present study, the high leaf tip chlorosis and necrosis observed in nonprimed seedlings indicated the poor growing conditions of ferruginous soil due to the toxic effect of iron. Ferruginous soils with high Fe and pH contents have been associated with chlorosis of emerging leaves, low photosynthetic properties, and stunted plant growth (Mahender et al., 2019), as observed in the ferruginous soil without priming in the present study. As shown in the results of the present study, priming of rice seeds with B. cereus strain GGBSU-1, P. mirabilis strain TL14-1, and K. variicola strain AUH-KAM-9 resulted in significant improvements.
The stunted growth of rice plants observed in nonprimed ferruginous soils (Fig. 4 and Table 5) may be a result of the limited amounts of bioavailable phosphorus and iron toxicity in these soils. Phosphorus is needed in large quantities in plants as it helps in the early stages of cell division; hence, phosphorus limitation can lead to the death of plants or delayed maturity (Uchida, 2000). The improvements in the number of tillers and reproductive tillers, 100-grain weight, and plant tissue water content can be attributed to the ability of the PSB to make important soil nutrients available for plants (Singh and Singh, 2011). The improved plant root architecture observed in FA showed the better root colonization capability of B. cereus strain GGBSU-1 compared with P. mirabilis strain TL14-1 and K. variicola strain AUH-KAM-9 (Fig. 13). Since bio-priming of rice seeds with B. cereus strain GGBSU-1 for 12 h (FA12) showed the highest rice growth and yield parameters even under ferruginous conditions, it can be recommended that biopriming of other important crops such as maize with PSB in ferruginous soils can be considered, to improve the productivity of grains in regions with iron toxic soil conditions.
Conclusions
To the best of our knowledge, this is the first report to investigate the possibilities of improving the growth and yield parameters of rice plants through bio-priming with PSB under high-iron soils with limited phosphorus bioavailability. The highest bacteria concentration in the rice seed endosperm was achieved after 12 h of priming. Similarly, the highest growth and yield properties of rice were achieved by priming for 12 h even under ferruginous soil conditions. Therefore, bio-priming of rice seeds with PSB might be considered a promising strategy for plant growth promotion under edaphic stress, especially priming with B. cereus strain GGBSU-1 for 12 h. Further studies on genomic and metabolomic aspects of these species in relation to their growth-promoting capabilities and phosphate-solubilizing efficiencies might be important to improve the strategies for sustainable crop production under different edaphic conditions.